Myogenic Vasoconstriction Requires Canonical Gq/11 Signaling of the Angiotensin II Type 1 Receptor
- PMID: 35132870
- PMCID: PMC9245832
- DOI: 10.1161/JAHA.121.022070
Myogenic Vasoconstriction Requires Canonical Gq/11 Signaling of the Angiotensin II Type 1 Receptor
Abstract
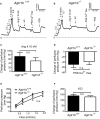
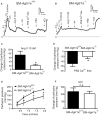
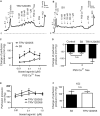
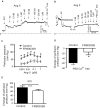
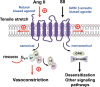
Background Blood pressure and tissue perfusion are controlled in part by the level of intrinsic (myogenic) arterial tone. However, many of the molecular determinants of this response are unknown. We previously found that mice with targeted disruption of the gene encoding the angiotensin II type 1a receptor (AT1AR) (Agtr1a), the major murine angiotensin II type 1 receptor (AT1R) isoform, showed reduced myogenic tone; however, uncontrolled genetic events (in this case, gene ablation) can lead to phenotypes that are difficult or impossible to interpret. Methods and Results We tested the mechanosensitive function of AT1R using tamoxifen-inducible smooth muscle-specific AT1aR knockout (smooth muscle-Agtr1a-/-) mice and studied downstream signaling cascades mediated by Gq/11 and/or β-arrestins. FR900359, Sar1Ile4Ile8-angiotensin II (SII), TRV120027 and TRV120055 were used as selective Gq/11 inhibitor and biased agonists to activate noncanonical β-arrestin and canonical Gq/11 signaling of the AT1R, respectively. Myogenic and Ang II-induced constrictions were diminished in the perfused renal vasculature, mesenteric and cerebral arteries of smooth muscle-Agtr1a-/- mice. Similar effects were observed in arteries of global mutant Agtr1a-/- but not Agtr1b-/- mice. FR900359 decreased myogenic tone and angiotensin II-induced constrictions whereas selective biased targeting of AT1R-β-arrestin signaling pathways had no effects. Conclusions This study demonstrates that myogenic arterial constriction requires Gq/11-dependent signaling pathways of mechanoactivated AT1R but not G protein-independent, noncanonical pathways in smooth muscle cells.
Keywords: angiotensin II type 1a receptor; arterial smooth muscle; biased ligands; myogenic vasoconstriction.
Figures



Comment in
-
Mechanosensitive Angiotensin II Receptor Signaling in Pressure-Induced Vasoconstriction.J Am Heart Assoc. 2022 Feb 15;11(4):e024740. doi: 10.1161/JAHA.121.024740. Epub 2022 Feb 12. J Am Heart Assoc. 2022. PMID: 35156384 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

