Tumor-penetrating peptide internalizing RGD enhances radiotherapy efficacy through reducing tumor hypoxia
- PMID: 35133063
- PMCID: PMC8990783
- DOI: 10.1111/cas.15295
Tumor-penetrating peptide internalizing RGD enhances radiotherapy efficacy through reducing tumor hypoxia
Abstract
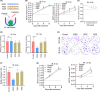
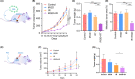
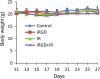
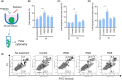
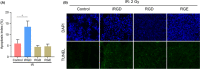
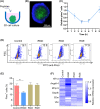
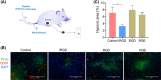
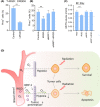
Resistance to irradiation (IR) remains a major therapeutic challenge in tumor radiotherapy. The development of novel tumor-specific radiosensitizers is crucial for effective radiotherapy against solid tumors. Here, we revealed that remodeling tumor tissue penetration via tumor-penetrating peptide internalizing arginine-glycine-aspartic acid RGD (iRGD) enhanced irradiation efficacy. The growth of 4T1 and CT26 multicellular tumor spheroids (MCTS) and tumors was delayed significantly by the treatment with IR and iRGD. Mechanistically, iRGD reduced hypoxia in MCTS and tumors, resulting in enhanced apoptosis after MCTS and tumors were treated with IR and iRGD. This is the first report that shows enhanced radiation efficacy by remodeling tumor-specific tissue penetration with iRGD, implying the potential clinical application of peptides in future tumor therapy.
Keywords: hypoxia; irradiation; multicellular tumor spheroids; radiosensitivity; tumor-penetrating peptides.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
Similar articles
-
Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates.Biomaterials. 2014 Oct;35(30):8735-47. doi: 10.1016/j.biomaterials.2014.06.042. Epub 2014 Jul 11. Biomaterials. 2014. PMID: 25023394
-
Tumor-penetrating peptide fused EGFR single-domain antibody enhances cancer drug penetration into 3D multicellular spheroids and facilitates effective gastric cancer therapy.J Control Release. 2015 Feb 28;200:188-200. doi: 10.1016/j.jconrel.2014.12.039. Epub 2014 Dec 30. J Control Release. 2015. PMID: 25553823 Free PMC article.
-
Recent advances in the tumor-penetrating peptide internalizing RGD for cancer treatment and diagnosis.Drug Dev Res. 2023 Jun;84(4):654-670. doi: 10.1002/ddr.22056. Epub 2023 Apr 5. Drug Dev Res. 2023. PMID: 36959702 Review.
-
Modification of IL-24 by tumor penetrating peptide iRGD enhanced its antitumor efficacy against non-small cell lung cancer.Int Immunopharmacol. 2019 May;70:125-134. doi: 10.1016/j.intimp.2019.02.027. Epub 2019 Feb 22. Int Immunopharmacol. 2019. PMID: 30798161
-
Exploration of tumor penetrating peptide iRGD as a potential strategy to enhance tumor penetration of cancer nanotherapeutics.Biochim Biophys Acta Rev Cancer. 2023 May;1878(3):188895. doi: 10.1016/j.bbcan.2023.188895. Epub 2023 Apr 8. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37037389 Review.
Cited by
-
Peptide therapeutics in the management of metastatic cancers.RSC Adv. 2022 Aug 2;12(33):21353-21373. doi: 10.1039/d2ra02062a. eCollection 2022 Jul 21. RSC Adv. 2022. PMID: 35975072 Free PMC article. Review.
-
Modification of erythrocytes by internalizing Arg-Gly-Asp (iRGD) in boosting the curative effect of radiotherapy for gastric carcinoma.J Gastrointest Oncol. 2022 Oct;13(5):2249-2258. doi: 10.21037/jgo-22-951. J Gastrointest Oncol. 2022. PMID: 36388665 Free PMC article.
-
Recent Advances in Mesoporous Silica Nanoparticles Delivering siRNA for Cancer Treatment.Pharmaceutics. 2023 Oct 17;15(10):2483. doi: 10.3390/pharmaceutics15102483. Pharmaceutics. 2023. PMID: 37896243 Free PMC article. Review.
References
-
- Carlson DJ, Yenice KM, Orton CG. Tumor hypoxia is an important mechanism of radioresistance in hypofractionated radiotherapy and must be considered in the treatment planning process. Med Phys. 2011;38:6347‐6350. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

