CHIP-associated mutant ASXL1 in blood cells promotes solid tumor progression
- PMID: 35133065
- PMCID: PMC8990791
- DOI: 10.1111/cas.15294
CHIP-associated mutant ASXL1 in blood cells promotes solid tumor progression
Abstract
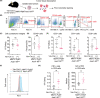
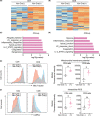
Clonal hematopoiesis of indeterminate potential (CHIP) is an age-associated phenomenon characterized by clonal expansion of blood cells harboring somatic mutations in hematopoietic genes, including DNMT3A, TET2, and ASXL1. Clinical evidence suggests that CHIP is highly prevalent and associated with poor prognosis in solid-tumor patients. However, whether blood cells with CHIP mutations play a causal role in promoting the development of solid tumors remained unclear. Using conditional knock-in mice that express CHIP-associated mutant Asxl1 (Asxl1-MT), we showed that expression of Asxl1-MT in T cells, but not in myeloid cells, promoted solid-tumor progression in syngeneic transplantation models. We also demonstrated that Asxl1-MT-expressing blood cells accelerated the development of spontaneous mammary tumors induced by MMTV-PyMT. Intratumor analysis of the mammary tumors revealed the reduced T-cell infiltration at tumor sites and programmed death receptor-1 (PD-1) upregulation in CD8+ T cells in MMTV-PyMT/Asxl1-MT mice. In addition, we found that Asxl1-MT induced T-cell dysregulation, including aberrant intrathymic T-cell development, decreased CD4/CD8 ratio, and naïve-memory imbalance in peripheral T cells. These results indicate that Asxl1-MT perturbs T-cell development and function, which contributes to creating a protumor microenvironment for solid tumors. Thus, our findings raise the possibility that ASXL1-mutated blood cells exacerbate solid-tumor progression in ASXL1-CHIP carriers.
Keywords: ASXL1; CHIP; T cell; mouse solid-tumor models; tumor immunity.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures




Similar articles
-
Mutant ASXL1 induces age-related expansion of phenotypic hematopoietic stem cells through activation of Akt/mTOR pathway.Nat Commun. 2021 Mar 23;12(1):1826. doi: 10.1038/s41467-021-22053-y. Nat Commun. 2021. PMID: 33758188 Free PMC article.
-
Clonal hematopoiesis and associated diseases: A review of recent findings.Cancer Sci. 2021 Oct;112(10):3962-3971. doi: 10.1111/cas.15094. Epub 2021 Aug 12. Cancer Sci. 2021. PMID: 34328684 Free PMC article. Review.
-
Clonal Hematopoiesis of Indeterminate Potential in Patients with Solid Tumor Malignancies.Cancer Res. 2022 Nov 15;82(22):4107-4113. doi: 10.1158/0008-5472.CAN-22-0985. Cancer Res. 2022. PMID: 36040522 Free PMC article.
-
Clonal hematopoiesis-related mutant ASXL1 promotes atherosclerosis in mice via dysregulated innate immunity.Nat Cardiovasc Res. 2024 Dec;3(12):1568-1583. doi: 10.1038/s44161-024-00579-w. Epub 2024 Dec 9. Nat Cardiovasc Res. 2024. PMID: 39653824
-
Aberrant histone modifications induced by mutant ASXL1 in myeloid neoplasms.Int J Hematol. 2019 Aug;110(2):179-186. doi: 10.1007/s12185-018-2563-7. Epub 2018 Dec 5. Int J Hematol. 2019. PMID: 30515738 Review.
Cited by
-
Obesity and Clonal Hematopoiesis of Indeterminate Potential: Allies in Cardiovascular Diseases and Malignancies.Life (Basel). 2023 Jun 10;13(6):1365. doi: 10.3390/life13061365. Life (Basel). 2023. PMID: 37374147 Free PMC article. Review.
-
Epigenome-wide DNA methylation association study of CHIP provides insight into perturbed gene regulation.Nat Commun. 2025 May 20;16(1):4678. doi: 10.1038/s41467-025-59333-w. Nat Commun. 2025. PMID: 40393957 Free PMC article.
-
Clonal hematopoiesis of indeterminate potential: contribution to disease and promising interventions.Mol Cell Biochem. 2025 Jul;480(7):4091-4106. doi: 10.1007/s11010-025-05261-8. Epub 2025 Mar 26. Mol Cell Biochem. 2025. PMID: 40140229 Review.
-
Epigenome-wide DNA Methylation Association Study of CHIP Provides Insight into Perturbed Gene Regulation.Res Sq [Preprint]. 2024 Jul 16:rs.3.rs-4656898. doi: 10.21203/rs.3.rs-4656898/v1. Res Sq. 2024. Update in: Nat Commun. 2025 May 20;16(1):4678. doi: 10.1038/s41467-025-59333-w. PMID: 39070619 Free PMC article. Updated. Preprint.
-
Clonal hematopoiesis in myeloid malignancies and solid tumors.Nat Cancer. 2025 Jul;6(7):1133-1144. doi: 10.1038/s43018-025-01014-0. Epub 2025 Jul 18. Nat Cancer. 2025. PMID: 40681796 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

