Ferroptosis in cancer and cancer immunotherapy
- PMID: 35133083
- PMCID: PMC8822596
- DOI: 10.1002/cac2.12250
Ferroptosis in cancer and cancer immunotherapy
Abstract
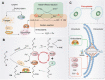
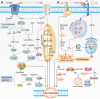
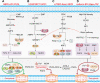
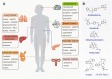
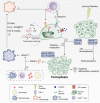
The hallmark of tumorigenesis is the successful circumvention of cell death regulation for achieving unlimited replication and immortality. Ferroptosis is a newly identified type of cell death dependent on lipid peroxidation which differs from classical programmed cell death in terms of morphology, physiology and biochemistry. The broad spectrum of injury and tumor tolerance are the main reasons for radiotherapy and chemotherapy failure. The effective rate of tumor immunotherapy as a new treatment method is less than 30%. Ferroptosis can be seen in radiotherapy, chemotherapy, and tumor immunotherapy; therefore, ferroptosis activation may be a potential strategy to overcome the drug resistance mechanism of traditional cancer treatments. In this review, the characteristics and causes of cell death by lipid peroxidation in ferroptosis are briefly described. In addition, the three metabolic regulations of ferroptosis and its crosstalk with classical signaling pathways are summarized. Collectively, these findings suggest the vital role of ferroptosis in immunotherapy based on the interaction of ferroptosis with tumor immunotherapy, chemotherapy and radiotherapy, thus, indicating the remarkable potential of ferroptosis in cancer treatment.
Keywords: cancer; ferroptosis; immunotherapy.
© 2022 The Authors. Cancer Communications published by John Wiley & Sons Australia, Ltd. on behalf of Sun Yat-sen University Cancer Center.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Ferroptosis: A New Promising Target for Lung Cancer Therapy.Oxid Med Cell Longev. 2021 Sep 25;2021:8457521. doi: 10.1155/2021/8457521. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34616505 Free PMC article. Review.
-
The mechanisms and therapeutic targets of ferroptosis in cancer.Expert Opin Ther Targets. 2021 Nov;25(11):965-986. doi: 10.1080/14728222.2021.2011206. Epub 2021 Dec 30. Expert Opin Ther Targets. 2021. PMID: 34821176
-
Ferroptosis is an effective strategy for cancer therapy.Med Oncol. 2024 Apr 23;41(5):124. doi: 10.1007/s12032-024-02317-5. Med Oncol. 2024. PMID: 38652406 Review.
-
Targeting the Macrophage-Ferroptosis Crosstalk: A Novel Insight into Tumor Immunotherapy.Front Biosci (Landmark Ed). 2022 Jun 27;27(7):203. doi: 10.31083/j.fbl2707203. Front Biosci (Landmark Ed). 2022. PMID: 35866391 Review.
-
Nanomedicine-mediated ferroptosis targeting strategies for synergistic cancer therapy.J Mater Chem B. 2023 Feb 8;11(6):1171-1190. doi: 10.1039/d2tb02161g. J Mater Chem B. 2023. PMID: 36650960 Review.
Cited by
-
Inflammatory Response in the Pathogenesis and Treatment of Hepatocellular Carcinoma: A Double-Edged Weapon.Int J Mol Sci. 2024 Jun 29;25(13):7191. doi: 10.3390/ijms25137191. Int J Mol Sci. 2024. PMID: 39000296 Free PMC article. Review.
-
Pancancer analysis reveals the role of disulfidptosis in predicting prognosis, immune infiltration and immunotherapy response in tumors.Medicine (Baltimore). 2023 Dec 29;102(52):e36830. doi: 10.1097/MD.0000000000036830. Medicine (Baltimore). 2023. PMID: 38206694 Free PMC article.
-
Cellular metabolism: A key player in cancer ferroptosis.Cancer Commun (Lond). 2024 Feb;44(2):185-204. doi: 10.1002/cac2.12519. Epub 2024 Jan 13. Cancer Commun (Lond). 2024. PMID: 38217522 Free PMC article. Review.
-
Construction of a ferroptosis-related eight gene signature for predicting the prognosis and immune infiltration of thyroid cancer.Front Endocrinol (Lausanne). 2022 Nov 4;13:997873. doi: 10.3389/fendo.2022.997873. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36407322 Free PMC article.
-
Cell Death Pathway Regulation by Functional Nanomedicines for Robust Antitumor Immunity.Adv Sci (Weinh). 2024 Jan;11(3):e2306580. doi: 10.1002/advs.202306580. Epub 2023 Nov 20. Adv Sci (Weinh). 2024. PMID: 37984863 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

