Axillary Adenopathy after COVID-19 Vaccine: No Reason to Delay Screening Mammogram
- PMID: 35133198
- PMCID: PMC8855316
- DOI: 10.1148/radiol.213227
Axillary Adenopathy after COVID-19 Vaccine: No Reason to Delay Screening Mammogram
Erratum in
-
Axillary Adenopathy after COVID-19 Vaccine: No Reason to Delay Screening Mammogram.Radiology. 2022 Sep;304(3):E57. doi: 10.1148/radiol.229015. Radiology. 2022. PMID: 35994402 Free PMC article. No abstract available.
Abstract
Online supplemental material is available for this article.
Conflict of interest statement
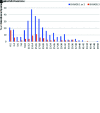
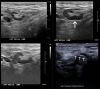
Figures

References
-
- Grimm L , Destounis S , Dogan B , et al. . SBI Recommendations for the Management of Axillary Adenopathy in Patients with Recent COVID-19 Vaccination . Society of Breast Imaging website . https://www.sbi-online.org/Portals/0/Position%20Statements/2021/SBI-reco.... Accessed December 16, 2021 .
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

