Clinical and Laboratory Approach to Diagnose COVID-19 Using Machine Learning
- PMID: 35133633
- PMCID: PMC8846962
- DOI: 10.1007/s12539-021-00499-4
Clinical and Laboratory Approach to Diagnose COVID-19 Using Machine Learning
Abstract
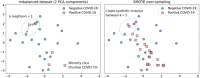
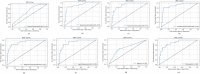
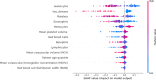
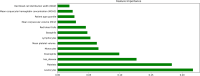
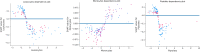
Coronavirus 2 (SARS-CoV-2), often known by the name COVID-19, is a type of acute respiratory syndrome that has had a significant influence on both economy and health infrastructure worldwide. This novel virus is diagnosed utilising a conventional method known as the RT-PCR (Reverse Transcription Polymerase Chain Reaction) test. This approach, however, produces a lot of false-negative and erroneous outcomes. According to recent studies, COVID-19 can also be diagnosed using X-rays, CT scans, blood tests and cough sounds. In this article, we use blood tests and machine learning to predict the diagnosis of this deadly virus. We also present an extensive review of various existing machine-learning applications that diagnose COVID-19 from clinical and laboratory markers. Four different classifiers along with a technique called Synthetic Minority Oversampling Technique (SMOTE) were used for classification. Shapley Additive Explanations (SHAP) method was utilized to calculate the gravity of each feature and it was found that eosinophils, monocytes, leukocytes and platelets were the most critical blood parameters that distinguished COVID-19 infection for our dataset. These classifiers can be utilized in conjunction with RT-PCR tests to improve sensitivity and in emergency situations such as a pandemic outbreak that might happen due to new strains of the virus. The positive results indicate the prospective use of an automated framework that could help clinicians and medical personnel diagnose and screen patients.
Keywords: Artificial Intelligence; Blood tests; COVID-19; Machine Learning; RT-PCR.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures

References
-
- WHO (2021) Coronavirus disease (covid-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 18 Dec 2021
-
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25:2000045. doi: 10.2807/1560-7917.es.2020.25.3.2000045. - DOI
-
- Döhla M, Boesecke C, Schulte B, Diegmann C, Sib E, Richter E, Eschbach-Bludau M, Aldabbagh S, Marx B, Eis-Hübinger AM, Schmithausen RM. Rapid point-of-care testing for SARS-CoV-2 in a community screening setting shows low sensitivity. Public Health. 2020;180:170–172. doi: 10.1016/j.puhe.2020.04.009. - DOI
-
- Burog AI, Yacapin CP, Maglente RR, Macalalad-Josue AA, Uy EJ, Dans AL, Dans LF. Should IgM/IgG rapid test kit be used in the diagnosis of COVID-19. Asia Pac Center Evid Based Healthc. 2020;4:1–12. doi: 10.47895/amp.v54i0.1558. - DOI
-
- Browning L, Colling R, Rakha E, Rajpoot N, Rittscher J, James JA, Salto-Tellez M, Snead DR, Verrill C. Digital pathology and artificial intelligence will be key to supporting clinical and academic cellular pathology through COVID-19 and future crises: the PathLAKE consortium perspective. J Clin Pathol. 2021;74(7):443–447. doi: 10.1136/jclinpath-2020-206854. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

