Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective
- PMID: 35133682
- PMCID: PMC8930624
- DOI: 10.1111/jpi.12790
Revisiting the role of melatonin in human melanocyte physiology: A skin context perspective
Abstract
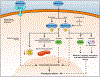
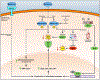
The evolutionarily ancient methoxyindoleamine, melatonin, has long perplexed investigators by its versatility of functions and mechanisms of action, which include the regulation of vertebrate pigmentation. Although first discovered through its potent skin-lightening effects in amphibians, melatonin's role in human skin and hair follicle pigmentation and its impact on melanocyte physiology remain unclear. Synthesizing our limited current understanding of this role, we specifically examine its impact on melanogenesis, oxidative biology, mitochondrial function, melanocyte senescence, and pigmentation-related clock gene activity, with emphasis on human skin, yet without ignoring instructive pointers from nonhuman species. Given the strict dependence of melanocyte functions on the epithelial microenvironment, we underscore that melanocyte responses to melatonin are best interrogated in a physiological tissue context. Current evidence suggests that melatonin and some of its metabolites inhibit both, melanogenesis (via reducing tyrosinase activity) and melanocyte proliferation by stimulating melatonin membrane receptors (MT1, MT2). We discuss whether putative melanogenesis-inhibitory effects of melatonin may occur via activation of Nrf2-mediated PI3K/AKT signaling, estrogen receptor-mediated and/or melanocortin-1 receptor- and cAMP-dependent signaling, and/or via melatonin-regulated changes in peripheral clock genes that regulate human melanogenesis, namely Bmal1 and Per1. Melatonin and its metabolites also accumulate in melanocytes where they exert net cyto- and senescence-protective as well as antioxidative effects by operating as free radical scavengers, stimulating the synthesis and activity of ROS scavenging enzymes and other antioxidants, promoting DNA repair, and enhancing mitochondrial function. We argue that it is clinically and biologically important to definitively clarify whether melanocyte cell culture-based observations translate into melatonin-induced pigmentary changes in a physiological tissue context, that is, in human epidermis and hair follicles ex vivo, and are confirmed by clinical trial results. After defining major open questions in this field, we close by suggesting how to begin answering them in clinically relevant, currently available preclinical in situ research models.
Keywords: hair follicle; human skin; melanocyte; melatonin; pigmentation.
© 2022 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Graying: gerontobiology of the hair follicle pigmentary unit.Exp Gerontol. 2001 Jan;36(1):29-54. doi: 10.1016/s0531-5565(00)00210-2. Exp Gerontol. 2001. PMID: 11162910 Review.
-
Hair cycle and hair pigmentation: dynamic interactions and changes associated with aging.Micron. 2004;35(3):193-200. doi: 10.1016/j.micron.2003.11.006. Micron. 2004. PMID: 15036274 Review.
-
The peripheral clock regulates human pigmentation.J Invest Dermatol. 2015 Apr;135(4):1053-1064. doi: 10.1038/jid.2014.442. Epub 2014 Oct 13. J Invest Dermatol. 2015. PMID: 25310406
-
Concentration-dependent stimulation of melanin production as well as melanocyte and keratinocyte proliferation by melatonin in human eyelid epidermis.Exp Dermatol. 2023 May;32(5):684-693. doi: 10.1111/exd.14740. Epub 2023 Jan 22. Exp Dermatol. 2023. PMID: 36601673
-
Post-tyrosinase inhibition of melanogenesis by melatonin in hair follicles in vitro.J Invest Dermatol. 1980 Jan;74(1):47-50. doi: 10.1111/1523-1747.ep12514608. J Invest Dermatol. 1980. PMID: 6766170
Cited by
-
Circular RNAs regulate parental gene expression: A new direction for molecular oncology research.Front Oncol. 2022 Aug 25;12:947775. doi: 10.3389/fonc.2022.947775. eCollection 2022. Front Oncol. 2022. PMID: 36091137 Free PMC article. Review.
-
Melatonin Exerts Prominent, Differential Epidermal and Dermal Anti-Aging Properties in Aged Human Eyelid Skin Ex Vivo.Int J Mol Sci. 2023 Nov 4;24(21):15963. doi: 10.3390/ijms242115963. Int J Mol Sci. 2023. PMID: 37958946 Free PMC article.
-
Research progress on peptides that inhibit melanin synthesis.Front Pharmacol. 2025 Jul 2;16:1610623. doi: 10.3389/fphar.2025.1610623. eCollection 2025. Front Pharmacol. 2025. PMID: 40672371 Free PMC article. Review.
-
Hair Follicles as a Critical Model for Monitoring the Circadian Clock.Int J Mol Sci. 2023 Jan 26;24(3):2407. doi: 10.3390/ijms24032407. Int J Mol Sci. 2023. PMID: 36768730 Free PMC article. Review.
-
Melatonin alleviates heat stress-induced testicular damage in dairy goats by inhibiting the PI3K/AKT signaling pathway.Stress Biol. 2022 Nov 14;2(1):47. doi: 10.1007/s44154-022-00068-9. Stress Biol. 2022. PMID: 37676539 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

