Metallointercalators-DNA Tetrahedron Supramolecular Self-Assemblies with Increased Serum Stability
- PMID: 35133785
- PMCID: PMC8926058
- DOI: 10.1021/acsnano.1c10084
Metallointercalators-DNA Tetrahedron Supramolecular Self-Assemblies with Increased Serum Stability
Abstract
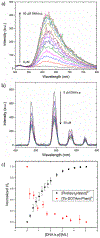
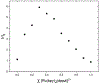
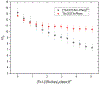
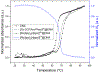
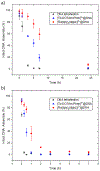
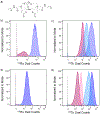
Self-assembly of metallointercalators into DNA nanocages is a rapid and facile approach to synthesize discrete bioinorganic host/guest structures with a high load of metal complexes. Turberfield's DNA tetrahedron can accommodate one intercalator for every two base pairs, which corresponds to 48 metallointercalators per DNA tetrahedron. The affinity of the metallointercalator for the DNA tetrahedron is a function of both the structure of the intercalating ligand and the overall charge of the complex, with a trend in affinity [Ru(bpy)2(dppz)]2+ > [Tb-DOTAm-Phen]3+ ≫ Tb-DOTA-Phen. Intercalation of the metal complex stabilizes the DNA tetrahedron, resulting in an increase of its melting temperature and, importantly, a significant increase in its stability in the presence of serum. [Ru(bpy)2(dppz)]2+, which has a greater affinity for DNA than [Tb-DOTAm-Phen]3+, increases the melting point and decreases degradation in serum to a greater extent than the TbIII complex. In the presence of Lipofectamine, the metallointercalator@DNA nanocage assemblies substantially increase the cell uptake of their respective metal complex. Altogether, the facile incorporation of a large number of metal complexes per assembly, the higher stability in serum, and the increased cell penetration of metallointercalator@DNA make these self-assemblies well-suited as metallodrugs.
Keywords: DNA tetrahedron; cell uptake; metallointercalator; serum stability; supramolecular self-assembly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

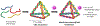

References
-
- Simoncelli S; Li Y; Cortés E; Maier SA Nanoscale Control of Molecular Self-Assembly Induced by Plasmonic Hot-Electron Dynamics. ACS Nano. 2018, 12, 2184–2192. - PubMed
-
- Whitesides GM; Mathias JP; Seto CT Molecular Self-Assembly and Nanochemistry: A Chemical Strategy for the Synthesis of Nanostructures. Science. 1991, 254, 1312–1319. - PubMed
-
- Zhang S Fabrication of Novel Biomaterials Through Molecular Self-Assembly. Nat. Biotechnol 2003, 21, 1171–1178. - PubMed
-
- Tabacchi G Supramolecular Organization in Confined Nanospaces. ChemPhysChem. 2018, 19, 1249–1297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

