Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes
- PMID: 35133977
- PMCID: PMC8970681
- DOI: 10.1172/JCI147719
Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes
Abstract
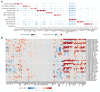
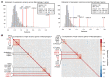
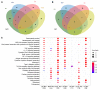
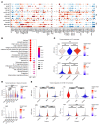
Trained immunity refers to the long-lasting memory traits of innate immunity. Recent studies have shown that trained immunity is orchestrated by sustained changes in epigenetic marks and metabolic pathways, leading to an altered transcriptional response to a second challenge. However, the potential heterogeneity of trained-immunity induction in innate immune cells has not been explored. In this study, we demonstrate cellular transcriptional programs in response to 4 different inducers of trained immunity in monocyte populations at single-cell resolution. Specifically, we identified 3 monocyte subpopulations upon the induction of trained immunity, and replicated these findings in an in vivo study. In addition, we found gene signatures consistent with these functional programs in patients with ulcerative colitis, sepsis, and COVID-19, suggesting the impact of trained-immunity programs in immune-mediated diseases.
Keywords: Immunology; Infectious disease; Innate immunity; Macrophages; Monocytes.
Figures



Comment in
-
Training can't always lead to Olympic macrophages.J Clin Invest. 2022 Apr 1;132(7):e158468. doi: 10.1172/JCI158468. J Clin Invest. 2022. PMID: 35362477 Free PMC article.

