Immunoglobulin A nephropathy is characterized by anticommensal humoral immune responses
- PMID: 35133979
- PMCID: PMC8983137
- DOI: 10.1172/jci.insight.141289
Immunoglobulin A nephropathy is characterized by anticommensal humoral immune responses
Abstract
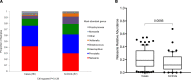
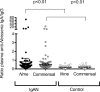
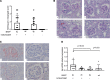
IgA nephropathy (IgAN) is a leading cause of kidney failure, yet little is known about the immunopathogenesis of this disease. IgAN is characterized by deposition of IgA in the kidney glomeruli, but the source and stimulus for IgA production are not known. Clinical and experimental data suggest a role for aberrant immune responses to mucosal microbiota in IgAN, and in some countries with high disease prevalence, tonsillectomy is regarded as standard-of-care therapy. To evaluate the relationship between microbiota and mucosal immune responses, we characterized the tonsil microbiota in patients with IgAN versus nonrelated household-matched control group participants and identified increased carriage of the genus Neisseria and elevated Neisseria-targeted serum IgA in IgAN patients. We reverse-translated these findings in experimental IgAN driven by BAFF overexpression in BAFF-transgenic mice rendered susceptible to Neisseria infection by introduction of a humanized CEACAM-1 transgene (B × hC-Tg). Colonization of B × hC-Tg mice with Neisseria yielded augmented levels of systemic Neisseria-specific IgA. Using a custom ELISPOT assay, we discovered anti-Neisseria-specific IgA-secreting cells within the kidneys of these mice. These findings suggest a role for cytokine-driven aberrant mucosal immune responses to oropharyngeal pathobionts, such as Neisseria, in the immunopathogenesis of IgAN. Furthermore, in the presence of excess BAFF, pathobiont-specific IgA can be produced in situ within the kidney.
Keywords: Chronic kidney disease; Cytokines; Immunoglobulins; Immunology; Nephrology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

