Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy
- PMID: 35133982
- PMCID: PMC8986065
- DOI: 10.1172/jci.insight.154395
Neurofilament proteins as a potential biomarker in chemotherapy-induced polyneuropathy
Abstract
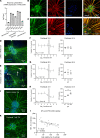
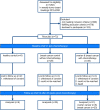
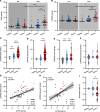
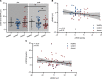
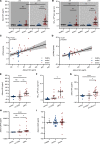
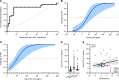
BACKGROUNDPaclitaxel chemotherapy frequently induces dose-limiting sensory axonal polyneuropathy. Given that sensory symptoms are challenging to assess objectively in clinical practice, an easily accessible biomarker for chemotherapy-induced polyneuropathy (CIPN) holds the potential to improve early diagnosis. Here, we describe neurofilament light chain (NFL), a marker for neuroaxonal damage, as a translational surrogate marker for CIPN.METHODSNFL concentrations were measured in an in vitro model of CIPN, exposing induced pluripotent stem cell-derived sensory neurons (iPSC-DSNs) to paclitaxel. Patients with breast or ovarian cancer undergoing paclitaxel chemotherapy, breast cancer control patients without chemotherapy, and healthy controls were recruited in a cohort study and examined before chemotherapy (V1) and after 28 weeks (V2, after chemotherapy). CIPN was assessed by the validated Total Neuropathy Score reduced (TNSr), which combines patient-reported symptoms with data from clinical examinations. Serum NFL (NFLs) concentrations were measured at both visits with single-molecule array technology.RESULTSNFL was released from iPSC-DSNs upon paclitaxel incubation in a dose- and time-dependent manner and was inversely correlated with iPSC-DSN viability. NFLs strongly increased in paclitaxel-treated patients with CIPN, but not in patients receiving chemotherapy without CIPN or controls, resulting in an 86% sensitivity and 87% specificity. An NFLs increase of +36 pg/mL from baseline was associated with a predicted CIPN probability of more than 0.5.CONCLUSIONNFLs was correlated with CIPN development and severity, which may guide neurotoxic chemotherapy in the future.TRIAL REGISTRATIONClinicalTrials.gov NCT02753036.FUNDINGDeutsche Forschungsgemeinschaft (EXC 257 NeuroCure), BMBF (Center for Stroke Research Berlin, 01 EO 0801), Animalfree Research, EU Horizon 2020 Innovative Medicines Initiative 2 Joint Undertaking (TransBioLine, 821283), Charité 3R - Replace - Reduce - Refine.
Keywords: Adult stem cells; Cancer; Neuroscience; Toxicology.
Figures