Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection
- PMID: 35133984
- PMCID: PMC8983145
- DOI: 10.1172/jci.insight.157342
Disulfiram inhibits neutrophil extracellular trap formation and protects rodents from acute lung injury and SARS-CoV-2 infection
Abstract
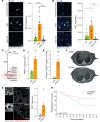
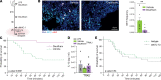
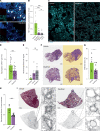
Severe acute lung injury has few treatment options and a high mortality rate. Upon injury, neutrophils infiltrate the lungs and form neutrophil extracellular traps (NETs), damaging the lungs and driving an exacerbated immune response. Unfortunately, no drug preventing NET formation has completed clinical development. Here, we report that disulfiram - an FDA-approved drug for alcohol use disorder - dramatically reduced NETs, increased survival, improved blood oxygenation, and reduced lung edema in a transfusion-related acute lung injury (TRALI) mouse model. We then tested whether disulfiram could confer protection in the context of SARS-CoV-2 infection, as NETs are elevated in patients with severe COVID-19. In SARS-CoV-2-infected golden hamsters, disulfiram reduced NETs and perivascular fibrosis in the lungs, and it downregulated innate immune and complement/coagulation pathways, suggesting that it could be beneficial for patients with COVID-19. In conclusion, an existing FDA-approved drug can block NET formation and improve disease course in 2 rodent models of lung injury for which treatment options are limited.
Keywords: COVID-19; Immunology; Innate immunity; Neutrophils.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

