Regional variability and genotypic and pharmacodynamic effects on PrP concentration in the CNS
- PMID: 35133987
- PMCID: PMC8986079
- DOI: 10.1172/jci.insight.156532
Regional variability and genotypic and pharmacodynamic effects on PrP concentration in the CNS
Abstract
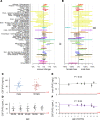
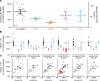
Prion protein (PrP) concentration controls the kinetics of prion replication and is a genetically and pharmacologically validated therapeutic target for prion disease. In order to evaluate PrP concentration as a pharmacodynamic biomarker and assess its contribution to known prion disease risk factors, we developed and validated a plate-based immunoassay reactive for PrP across 6 species of interest and applicable to brain and cerebrospinal fluid (CSF). PrP concentration varied dramatically across different brain regions in mice, cynomolgus macaques, and humans. PrP expression did not appear to contribute to the known risk factors of age, sex, or common PRNP genetic variants. CSF PrP was lowered in the presence of rare pathogenic PRNP variants, with heterozygous carriers of P102L displaying 55%, and D178N just 31%, of the CSF PrP concentration of mutation-negative controls. In rodents, pharmacologic reduction of brain Prnp RNA was reflected in brain parenchyma PrP and, in turn in CSF PrP, validating CSF as a sampling compartment for the effect of PrP-lowering therapy. Our findings support the use of CSF PrP as a pharmacodynamic biomarker for PrP-lowering drugs and suggest that relative reduction from individual baseline CSF PrP concentration may be an appropriate marker for target engagement.
Keywords: Neurodegeneration; Neuroscience; Prions; Therapeutics.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

