A SARS-CoV-2 variant elicits an antibody response with a shifted immunodominance hierarchy
- PMID: 35134084
- PMCID: PMC8856557
- DOI: 10.1371/journal.ppat.1010248
A SARS-CoV-2 variant elicits an antibody response with a shifted immunodominance hierarchy
Abstract
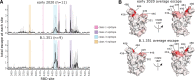
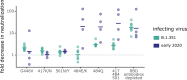
Many SARS-CoV-2 variants have mutations at key sites targeted by antibodies. However, it is unknown if antibodies elicited by infection with these variants target the same or different regions of the viral spike as antibodies elicited by earlier viral isolates. Here we compare the specificities of polyclonal antibodies produced by humans infected with early 2020 isolates versus the B.1.351 variant of concern (also known as Beta or 20H/501Y.V2), which contains mutations in multiple key spike epitopes. The serum neutralizing activity of antibodies elicited by infection with both early 2020 viruses and B.1.351 is heavily focused on the spike receptor-binding domain (RBD). However, within the RBD, B.1.351-elicited antibodies are more focused on the "class 3" epitope spanning sites 443 to 452, and neutralization by these antibodies is notably less affected by mutations at residue 484. Our results show that SARS-CoV-2 variants can elicit polyclonal antibodies with different immunodominance hierarchies.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: J.D.B. consults for Moderna and Flagship Labs 77 on topics related to viral evolution, and has the potential to receive a share of IP revenue as an inventor on a Fred Hutch optioned technology/patent (application WO2020006494) related to deep mutational scanning of viral proteins. A.J.G, T.N.S., and J.D.B have the potential to receive a share of IP revenue as an inventor on a Fred Hutch optioned technology related to deep mutational scanning of the receptor-binding domain of SARS-CoV-2 Spike protein. H.Y.C. is a consultant for Merck, Pfizer, Ellume, and the Bill and Melinda Gates Foundation and has received support from Cepheid and Sanofi-Pasteur. D.V. is named as an inventor on a patent application filed by the University of Washington related to SARS-CoV-2 vaccines and has received an unrelated sponsored research agreement from Vir Biotechnology Inc. D.C. is an employee of and may hold shares in Vir Biotechnology. The other authors declare no competing interests.
Figures



Update of
-
A SARS-CoV-2 variant elicits an antibody response with a shifted immunodominance hierarchy.bioRxiv [Preprint]. 2021 Oct 13:2021.10.12.464114. doi: 10.1101/2021.10.12.464114. bioRxiv. 2021. Update in: PLoS Pathog. 2022 Feb 8;18(2):e1010248. doi: 10.1371/journal.ppat.1010248. PMID: 34671768 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

