Pharmacological and genetic activation of cAMP synthesis disrupts cholesterol utilization in Mycobacterium tuberculosis
- PMID: 35134095
- PMCID: PMC8856561
- DOI: 10.1371/journal.ppat.1009862
Pharmacological and genetic activation of cAMP synthesis disrupts cholesterol utilization in Mycobacterium tuberculosis
Abstract
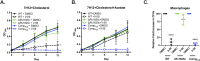
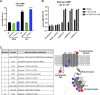
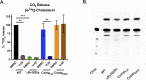
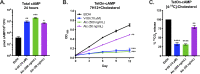
There is a growing appreciation for the idea that bacterial utilization of host-derived lipids, including cholesterol, supports Mycobacterium tuberculosis (Mtb) pathogenesis. This has generated interest in identifying novel antibiotics that can disrupt cholesterol utilization by Mtb in vivo. Here we identify a novel small molecule agonist (V-59) of the Mtb adenylyl cyclase Rv1625c, which stimulates 3', 5'-cyclic adenosine monophosphate (cAMP) synthesis and inhibits cholesterol utilization by Mtb. Similarly, using a complementary genetic approach that induces bacterial cAMP synthesis independent of Rv1625c, we demonstrate that inducing cAMP synthesis is sufficient to inhibit cholesterol utilization in Mtb. Although the physiological roles of individual adenylyl cyclase enzymes in Mtb are largely unknown, here we demonstrate that the transmembrane region of Rv1625c is required during cholesterol metabolism. Finally, the pharmacokinetic properties of Rv1625c agonists have been optimized, producing an orally-available Rv1625c agonist that impairs Mtb pathogenesis in infected mice. Collectively, this work demonstrates a role for Rv1625c and cAMP signaling in controlling cholesterol metabolism in Mtb and establishes that cAMP signaling can be pharmacologically manipulated for the development of new antibiotic strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


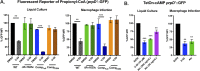
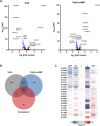
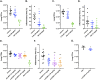
References
-
- World Health Organization. Global Tuberculosis Report 2019. (World Health Organization, Geneva, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

