Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia
- PMID: 35134115
- PMCID: PMC9006280
- DOI: 10.1182/bloodadvances.2021006700
Fludarabine exposure predicts outcome after CD19 CAR T-cell therapy in children and young adults with acute leukemia
Abstract
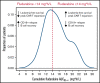
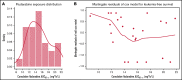
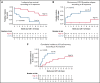
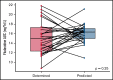
The addition of fludarabine to cyclophosphamide as a lymphodepleting regimen prior to CD19 chimeric antigen receptor (CAR) T-cell therapy significantly improved outcomes in patients with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL). Fludarabine exposure, previously shown to be highly variable when dosing is based on body surface area (BSA), is a predictor for survival in allogeneic hematopoietic cell transplantation (allo-HCT). Hence, we hypothesized that an optimal exposure of fludarabine might be of clinical importance in CD19 CAR T-cell treatment. We examined the effect of cumulative fludarabine exposure during lymphodepletion, defined as concentration-time curve (AUC), on clinical outcome and lymphocyte kinetics. A retrospective analysis was conducted with data from 26 patients receiving tisagenlecleucel for r/r B-ALL. Exposure of fludarabine was shown to be a predictor for leukemia-free survival (LFS), B-cell aplasia, and CD19-positive relapse following CAR T-cell infusion. Minimal event probability was observed at a cumulative fludarabine AUCT0-∞ ≥14 mg*h/L, and underexposure was defined as an AUCT0-∞ <14 mg*h/L. In the underexposed group, the median LFS was 1.8 months, and the occurrence of CD19-positive relapse within 1 year was 100%, which was higher compared with the group with an AUCT0-∞ ≥14 mg*h/L (12.9 months; P < .001; and 27.4%; P = .0001, respectively). Furthermore, the duration of B-cell aplasia within 6 months was shorter in the underexposed group (77.3% vs 37.3%; P = .009). These results suggest that optimizing fludarabine exposure may have a relevant impact on LFS following CAR T-cell therapy, which needs to be validated in a prospective clinical trial.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

