Placental sFlt-1 Gene Delivery in Early Primate Pregnancy Suppresses Uterine Spiral Artery Remodeling
- PMID: 35134145
- PMCID: PMC8896163
- DOI: 10.1210/endocr/bqac012
Placental sFlt-1 Gene Delivery in Early Primate Pregnancy Suppresses Uterine Spiral Artery Remodeling
Abstract
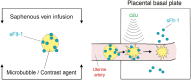
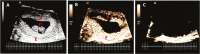
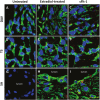
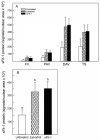
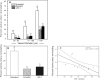
Uterine spiral artery remodeling (SAR) is essential for promoting placental perfusion and fetal development. A defect in SAR results in placental ischemia and increase in placental expression and serum levels of the soluble fms-like tyrosine kinase-1 (sFlt-1) receptor that binds to and suppresses vascular endothelial growth factor (VEGF) bioavailability, thereby leading to maternal vascular dysfunction. We have established a nonhuman primate model of impaired SAR and maternal vascular dysfunction by prematurely elevating estradiol levels in early baboon pregnancy. However, it is unknown whether this primate model of defective SAR involves an increase in placental expression of sFlt-1, which may suppress VEGF bioavailability and thus SAR in the first trimester. Therefore, to establish the role of sFlt-1 in early pregnancy, SAR was quantified in baboons treated on days 25 through 59 of gestation (term = 184 days) with estradiol or with the sFlt-1 gene targeted selectively to the placental basal plate by ultrasound-mediated/microbubble-facilitated gene delivery technology. Placental basal plate sFlt-1 protein expression was 2-fold higher (P < 0.038) and the level of SAR for vessels > 25 µm in diameter was 72% and 63% lower (P < 0.01), respectively, in estradiol-treated and sFlt-1 gene-treated baboons than in untreated animals. In summary, prematurely elevating estradiol levels or sFlt-1 gene delivery increased placental basal plate sFlt-1 protein expression and suppressed SAR in early baboon pregnancy. This study makes the novel discovery that in elevated levels sFlt-1 has a role both in suppressing SAR in early primate pregnancy and maternal vascular endothelial function in late gestation.
Keywords: 1 primate spiral artery remodeling; sFlt.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

