Cytomegalovirus Latent Infection is Associated with an Increased Risk of COVID-19-Related Hospitalization
- PMID: 35134186
- PMCID: PMC8905965
- DOI: 10.1093/infdis/jiac020
Cytomegalovirus Latent Infection is Associated with an Increased Risk of COVID-19-Related Hospitalization
Abstract
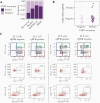
Some risk factors for severe coronavirus disease 2019 (COVID-19) have been identified, including age, race, and obesity. However, 20%-50% of severe cases occur in the absence of these factors. Cytomegalovirus (CMV) is a herpesvirus that infects about 50% of all individuals worldwide and is among the most significant nongenetic determinants of immune system. We hypothesized that latent CMV infection might influence the severity of COVID-19. Our analyses demonstrate that CMV seropositivity is associated with more than twice the risk of hospitalization due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Immune profiling of blood and CMV DNA quantitative polymerase chain reaction in a subset of patients for whom respiratory tract samples were available revealed altered T-cell activation profiles in absence of extensive CMV replication in the upper respiratory tract. These data suggest a potential role for CMV-driven immune perturbations in affecting the outcome of SARS-CoV-2 infection and may have implications for the discrepancies in COVID-19 severity between different human populations.
Keywords: CMV; COVID-19; EMRA T cells; immune profiling; risk factor.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures




References
-
- World Health Organization. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. 2021:1–25. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

