Structural Forces in Ionic Liquids: The Role of Ionic Size Asymmetry
- PMID: 35134297
- PMCID: PMC9007453
- DOI: 10.1021/acs.jpcb.1c09441
Structural Forces in Ionic Liquids: The Role of Ionic Size Asymmetry
Abstract
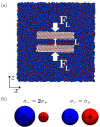
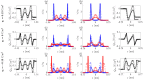
Ionic liquids (ILs) are charged fluids composed of anions and cations of different size and shape. The ordering of charge and density in ILs confined between charged interfaces underlies numerous applications of IL electrolytes. Here, we analyze the screening behavior and the resulting structural forces of a representative IL confined between two charge-varied plates. Using both molecular dynamics simulations and a continuum theory, we contrast the screening features of a more-realistic asymmetric system and a less-realistic symmetric one. The ionic size asymmetry plays a nontrivial role in charge screening, affecting both the ionic density profiles and the disjoining pressure distance dependence. Ionic systems with size asymmetry are stronger coupled systems, and this manifests itself both in their response to the electrode polarization and spontaneous structure formation at the interface. Analytical expressions for decay lengths of the disjoining pressure are obtained in agreement with the pressure profiles computed from molecular dynamics simulations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Palacio M.; Bhushan B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. 10.1007/s11249-010-9671-8. - DOI
LinkOut - more resources
Full Text Sources

