Effect of Nicotinamide in Skin Cancer and Actinic Keratoses Chemoprophylaxis, and Adverse Effects Related to Nicotinamide: A Systematic Review and Meta-Analysis
- PMID: 35134311
- PMCID: PMC9125143
- DOI: 10.1177/12034754221078201
Effect of Nicotinamide in Skin Cancer and Actinic Keratoses Chemoprophylaxis, and Adverse Effects Related to Nicotinamide: A Systematic Review and Meta-Analysis
Abstract
Background: Oral nicotinamide is recommended in individuals with a field of cancerization or with ≥1 previous cutaneous squamous cell carcinoma (cSCC).
Objective: To evaluate the effect of nicotinamide in prevention of skin cancers.
Methods: We conducted a systematic review and meta-analysis of randomized controlled trials to evaluate the effect of nicotinamide. We used Medline, EMBASE, CENTRAL, and Web of Science databases from their inception to October 2020 to search the following concepts: "nicotinamide"; "randomized controlled trial" (validated filters). Two independent reviewers screened titles and abstracts for intervention and study design before searching full texts for eligibility criteria. To be eligible, ≥1 outcome had to be covered. We used a standardized collection grid to complete data extraction in duplicate. The primary outcome was skin cancers (all types). Secondary outcomes were basal cell carcinomas (BCCs); cSCCs; actinic keratoses; melanomas; digestive, cutaneous, and biochemical adverse effects (AEs). Subgroup analyses were planned a priori.
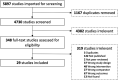
Results: We screened 4730 citations and found 29 trials (3039 patients) meeting inclusion criteria. Nicotinamide was associated with a significant reduction in skin cancers compared to control (rate ratio 0.50 (95% CI, 0.29-0.85; I 2 = 64%; 552 patients; 5 trials); moderate strength of the evidence). Heterogeneity was explained by risk of bias. Nicotinamide was associated with a significant reduction in BCCs and cSCCs, and increased risk of digestive AEs.
Conclusion: Oral nicotinamide should be considered in healthy patients or organ transplant recipients with history of skin cancer (GRADE: weak recommendation; moderate-quality evidence), in particular of BCC and cSCC.
Keywords: actinic keratosis; adverse effect; basal cell carcinoma; chemoprevention; chemoprophylaxis; cutaneous squamous cell carcinoma; melanoma; niacinamide; nicotinamide; oncology; skin cancer.
Conflict of interest statement
Figures





References
-
- Statistics CCSsSCoC . Canadian cancer statistics. Canadian Cancer Society; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

