Certolizumab Pegol Use in the Treatment of Moderate-to-Severe Psoriasis: Real-World Data From Two Canadian Centers
- PMID: 35134313
- PMCID: PMC9125136
- DOI: 10.1177/12034754221078203
Certolizumab Pegol Use in the Treatment of Moderate-to-Severe Psoriasis: Real-World Data From Two Canadian Centers
Abstract
Background: Certolizumab pegol (CZP) is a TNF-ɑ inhibitor used to treat moderate-to-severe plaque psoriasis (PsO) in adult patients, including women of childbearing potential (WOCBP) and patients with psoriatic arthritis (PsA). There are currently limited real-world data on CZP for treatment of PsO.
Objectives: To examine the use of CZP for treatment of PsO in clinical practice at two dermatology clinics in Canada.
Methods: We conducted a retrospective chart analysis of 59 patients with moderate-to-severe psoriasis receiving CZP. Clinical efficacy was measured using the Psoriasis Area and Severity Index (PASI), Body Surface Area (BSA), and Physician Global Assessment (PGA). Drug survival was analyzed using Kaplan-Meier plots.
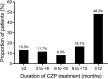
Results: Of the 59 patients, 36 (61%) were female, of whom 23 (63.9%) were WOCBP. Twenty-three (39.0%) patients received CZP as their first biologic treatment. The main reasons for choosing CZP were its efficacy in both PsO and PsA, and for WOCBP due to little or no cross-placental transfer. Improvement of symptoms was observed after 3 months of treatment and was maintained for the 12-month analysis period. After 12 months of treatment, the patients' mean PASI score decreased from 13.0 (±5.8) at baseline to 2.3 (±4.3), mean BSA score from 13.1% (±6.7%) to 1.7% (±2.6%), and mean PGA score from 3.0 (±0.6) to 0.8 (±0.6). Overall CZP drug survival rate was 76.3% at 12 months, with no difference between biologic-naive and biologic-experienced patients.
Conclusions: CZP was effective and well tolerated in this cohort of patients with moderate-to-severe PsO in a real-world setting.
Keywords: TNF-alpha inhibitor; biologics; certolizumab pegol; psoriasis; real-world data.
Conflict of interest statement
Figures


Similar articles
-
[Translated article] Risk-sharing agreement based on health outcomes for the treatment of moderate-severe psoriasis with certolizumab pegol.Farm Hosp. 2024 Mar-Apr;48(2):T51-T56. doi: 10.1016/j.farma.2023.11.004. Epub 2023 Dec 25. Farm Hosp. 2024. PMID: 38148255 English, Spanish.
-
Clinical efficacy and safety of certolizumab pegol in cutaneous symptoms on psoriasis in patients with psoriatic arthritis: A retrospective analysis in real life.Dermatol Ther. 2020 May;33(3):e13409. doi: 10.1111/dth.13409. Epub 2020 May 12. Dermatol Ther. 2020. PMID: 32291887
-
Safety and efficacy of certolizumab pegol in a real-life cohort of patients with psoriasis and psoriatic arthritis.J Dermatolog Treat. 2020 Nov;31(7):692-697. doi: 10.1080/09546634.2019.1605143. Epub 2019 May 6. J Dermatolog Treat. 2020. PMID: 30963798
-
Certolizumab pegol for the treatment of psoriatic arthritis and plaque psoriasis.Expert Rev Clin Immunol. 2020 Feb;16(2):119-128. doi: 10.1080/1744666X.2020.1713754. Epub 2020 Jan 22. Expert Rev Clin Immunol. 2020. PMID: 31917928 Review.
-
Certolizumab Pegol: A Review in Moderate to Severe Plaque Psoriasis.BioDrugs. 2020 Apr;34(2):235-244. doi: 10.1007/s40259-020-00416-z. BioDrugs. 2020. PMID: 32207094 Review.
Cited by
-
The roles of T cells in psoriasis.Front Immunol. 2023 Oct 24;14:1081256. doi: 10.3389/fimmu.2023.1081256. eCollection 2023. Front Immunol. 2023. PMID: 37942312 Free PMC article. Review.
-
Certolizumab Pegol for the Treatment of Plaque Psoriasis in Routine Clinical Practice: One-Year Results from the CIMREAL Study.Dermatol Ther (Heidelb). 2024 Aug;14(8):2077-2092. doi: 10.1007/s13555-024-01210-3. Epub 2024 Jun 27. Dermatol Ther (Heidelb). 2024. PMID: 38937404 Free PMC article.
-
Plant-Based Foods for Chronic Skin Diseases: A Focus on the Mediterranean Diet.Curr Nutr Rep. 2025 Mar 6;14(1):42. doi: 10.1007/s13668-025-00632-5. Curr Nutr Rep. 2025. PMID: 40048018 Free PMC article. Review.
-
Tapinarof Nanogels as a Promising Therapeutic Approach.Pharmaceutics. 2025 Jun 1;17(6):731. doi: 10.3390/pharmaceutics17060731. Pharmaceutics. 2025. PMID: 40574044 Free PMC article. Review.
-
Childhood guttate psoriasis: an updated review.Drugs Context. 2023 Oct 23;12:2023-8-2. doi: 10.7573/dic.2023-8-2. eCollection 2023. Drugs Context. 2023. PMID: 37908643 Free PMC article. Review.
References
-
- Reich K., Ortonne J-P., Gottlieb AB. et al.. Successful treatment of moderate to severe plaque psoriasis with the PEGylated Fab’ certolizumab pegol: results of a phase II randomized, placebo-controlled trial with a re-treatment extension. Br J Dermatol. 2012;167(1):180-190.10.1111/j.1365-2133.2012.10941.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

