Distinct Cellular Tropism and Immune Responses to Alphavirus Infection
- PMID: 35134315
- PMCID: PMC10350340
- DOI: 10.1146/annurev-immunol-101220-014952
Distinct Cellular Tropism and Immune Responses to Alphavirus Infection
Abstract
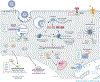
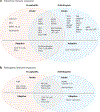
Alphaviruses are emerging and reemerging viruses that cause disease syndromes ranging from incapacitating arthritis to potentially fatal encephalitis. While infection by arthritogenic and encephalitic alphaviruses results in distinct clinical manifestations, both virus groups induce robust innate and adaptive immune responses. However, differences in cellular tropism, type I interferon induction, immune cell recruitment, and B and T cell responses result in differential disease progression and outcome. In this review, we discuss aspects of immune responses that contribute to protective or pathogenic outcomes after alphavirus infection.
Keywords: alphavirus; arbovirus; immunity; mouse models; pathogenesis; tropism.
Figures



Similar articles
-
Host responses to alphavirus infection.Immunol Rev. 2008 Oct;225:27-45. doi: 10.1111/j.1600-065X.2008.00670.x. Immunol Rev. 2008. PMID: 18837774 Review.
-
Innate immune control of alphavirus infection.Curr Opin Virol. 2018 Feb;28:53-60. doi: 10.1016/j.coviro.2017.11.006. Epub 2017 Nov 22. Curr Opin Virol. 2018. PMID: 29175515 Free PMC article. Review.
-
Animal models of alphavirus infection and human disease.Adv Virus Res. 2022;113:25-88. doi: 10.1016/bs.aivir.2022.07.001. Epub 2022 Aug 22. Adv Virus Res. 2022. PMID: 36307168 Review.
-
Immunopathogenesis of alphaviruses.Adv Virus Res. 2020;107:315-382. doi: 10.1016/bs.aivir.2020.06.002. Epub 2020 Jul 8. Adv Virus Res. 2020. PMID: 32711733 Free PMC article.
-
The Interferon-Stimulated Gene IFITM3 Restricts Infection and Pathogenesis of Arthritogenic and Encephalitic Alphaviruses.J Virol. 2016 Sep 12;90(19):8780-94. doi: 10.1128/JVI.00655-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440901 Free PMC article.
Cited by
-
Entry receptor LDLRAD3 is required for Venezuelan equine encephalitis virus peripheral infection and neurotropism leading to pathogenesis in mice.Cell Rep. 2023 Aug 29;42(8):112946. doi: 10.1016/j.celrep.2023.112946. Epub 2023 Aug 8. Cell Rep. 2023. PMID: 37556325 Free PMC article.
-
Chikungunya and Mayaro Viruses Induce Chronic Skeletal Muscle Atrophy Triggered by Pro-Inflammatory and Oxidative Response.Int J Mol Sci. 2024 Aug 16;25(16):8909. doi: 10.3390/ijms25168909. Int J Mol Sci. 2024. PMID: 39201595 Free PMC article.
-
Obesity-Associated Changes in Immune Cell Dynamics During Alphavirus Infection Revealed by Single Cell Transcriptomic Analysis.bioRxiv [Preprint]. 2024 Oct 13:2024.10.10.617696. doi: 10.1101/2024.10.10.617696. bioRxiv. 2024. PMID: 39416014 Free PMC article. Preprint.
-
N-acetyltransferase 10 regulates alphavirus replication via N4-acetylcytidine (ac4C) modification of the lymphocyte antigen six family member E (LY6E) mRNA.J Virol. 2024 Jan 23;98(1):e0135023. doi: 10.1128/jvi.01350-23. Epub 2024 Jan 3. J Virol. 2024. PMID: 38169284 Free PMC article.
-
Lack of pathogenic involvement of CCL4 and its receptor CCR5 in arthritogenic alphavirus disease.bioRxiv [Preprint]. 2024 Aug 3:2024.07.31.606106. doi: 10.1101/2024.07.31.606106. bioRxiv. 2024. Update in: Immunohorizons. 2025 May 30;9(7):vlaf022. doi: 10.1093/immhor/vlaf022. PMID: 39131287 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

