Striatal dopamine signals are region specific and temporally stable across action-sequence habit formation
- PMID: 35134325
- PMCID: PMC8926842
- DOI: 10.1016/j.cub.2021.12.027
Striatal dopamine signals are region specific and temporally stable across action-sequence habit formation
Abstract
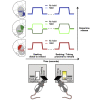
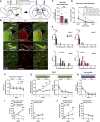
Habits are automatic, inflexible behaviors that develop slowly with repeated performance. Striatal dopamine signaling instantiates this habit-formation process, presumably region specifically and via ventral-to-dorsal and medial-to-lateral signal shifts. Here, we quantify dopamine release in regions implicated in these presumed shifts (ventromedial striatum [VMS], dorsomedial striatum [DMS], and dorsolateral striatum [DLS]) in rats performing an action-sequence task and characterize habit development throughout a 10-week training. Surprisingly, all regions exhibited stable dopamine dynamics throughout habit development. VMS and DLS signals did not differ between habitual and non-habitual animals, but DMS dopamine release increased during action-sequence initiation and decreased during action-sequence completion in habitual rats, whereas non-habitual rats showed opposite effects. Consistently, optogenetic stimulation of DMS dopamine release accelerated habit formation. Thus, we demonstrate that dopamine signals do not shift regionally during habit formation and that dopamine in DMS, but not VMS or DLS, determines habit bias, attributing "habit functions" to a region previously associated exclusively with non-habitual behavior.
Keywords: action repetition; automated behavior; basal ganglia; behavior; dopamine; goal-directed behavior; habit formation; habits; rat; striatum.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



Similar articles
-
Characterization of striatal dopamine projections across striatal subregions in behavioral flexibility.Eur J Neurosci. 2023 Dec;58(12):4466-4486. doi: 10.1111/ejn.15910. Epub 2023 Jan 22. Eur J Neurosci. 2023. PMID: 36617434 Free PMC article.
-
Optogenetic stimulation of striatal patches modifies habit formation and inhibits dopamine release.Sci Rep. 2021 Oct 6;11(1):19847. doi: 10.1038/s41598-021-99350-5. Sci Rep. 2021. PMID: 34615966 Free PMC article.
-
Complementary Control over Habits and Behavioral Vigor by Phasic Activity in the Dorsolateral Striatum.J Neurosci. 2020 Mar 4;40(10):2139-2153. doi: 10.1523/JNEUROSCI.1313-19.2019. Epub 2020 Jan 22. J Neurosci. 2020. PMID: 31969469 Free PMC article.
-
Interfacing behavioral and neural circuit models for habit formation.J Neurosci Res. 2020 Jun;98(6):1031-1045. doi: 10.1002/jnr.24581. Epub 2020 Jan 8. J Neurosci Res. 2020. PMID: 31916623 Free PMC article. Review.
-
Striatal D2: Where habits and newly learned actions meet.Learn Behav. 2022 Sep;50(3):267-268. doi: 10.3758/s13420-022-00526-4. Epub 2022 May 26. Learn Behav. 2022. PMID: 35618985 Review.
Cited by
-
Pallidal prototypic neuron and astrocyte activities regulate flexible reward-seeking behaviors.bioRxiv [Preprint]. 2025 Feb 10:2025.02.10.637554. doi: 10.1101/2025.02.10.637554. bioRxiv. 2025. PMID: 39990452 Free PMC article. Preprint.
-
A role for the dorsolateral striatum in prospective action control.iScience. 2024 May 21;27(6):110044. doi: 10.1016/j.isci.2024.110044. eCollection 2024 Jun 21. iScience. 2024. PMID: 38883824 Free PMC article.
-
Pre-existing visual responses in a projection-defined dopamine population explain individual learning trajectories.Curr Biol. 2024 Nov 18;34(22):5349-5358.e6. doi: 10.1016/j.cub.2024.09.045. Epub 2024 Oct 16. Curr Biol. 2024. PMID: 39413788 Free PMC article.
-
Mesolimbic dopamine release conveys causal associations.Science. 2022 Dec 23;378(6626):eabq6740. doi: 10.1126/science.abq6740. Epub 2022 Dec 23. Science. 2022. PMID: 36480599 Free PMC article.
-
Nucleus accumbens and dorsal medial striatal dopamine and neural activity are essential for action sequence performance.Eur J Neurosci. 2024 Jan;59(2):220-237. doi: 10.1111/ejn.16210. Epub 2023 Dec 13. Eur J Neurosci. 2024. PMID: 38093522 Free PMC article.
References
-
- Adams C.D. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q. J. Exp. Psychol. B. 1982;34:77–98.
-
- Dickinson A., Nicholas D.J., Adams C.D. The effect of the instrumental training contingency on susceptibility to reinforcer devaluation. Q. J. Exp. Psychol. B. 1983;35:35–51.
-
- Dickinson A. Actions and habits: the development of behavioural autonomy. Philos. Trans. R. Soc. Lond. B. 1985;308:67–78.
-
- Packard M.G., McGaugh J.L. Double dissociation of fornix and caudate nucleus lesions on acquisition of two water maze tasks: further evidence for multiple memory systems. Behav. Neurosci. 1992;106:439–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

