Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles
- PMID: 35134856
- PMCID: PMC10022859
- DOI: 10.1093/cvr/cvac012
Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles
Abstract
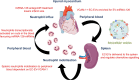
Aims: Acute myocardial infarction rapidly increases blood neutrophils (<2 h). Release from bone marrow, in response to chemokine elevation, has been considered their source, but chemokine levels peak up to 24 h after injury, and after neutrophil elevation. This suggests that additional non-chemokine-dependent processes may be involved. Endothelial cell (EC) activation promotes the rapid (<30 min) release of extracellular vesicles (EVs), which have emerged as an important means of cell-cell signalling and are thus a potential mechanism for communicating with remote tissues.
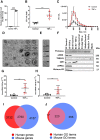
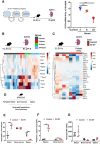
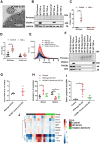
Methods and results: Here, we show that injury to the myocardium rapidly mobilizes neutrophils from the spleen to peripheral blood and induces their transcriptional activation prior to arrival at the injured tissue. Time course analysis of plasma-EV composition revealed a rapid and selective increase in EVs bearing VCAM-1. These EVs, which were also enriched for miRNA-126, accumulated preferentially in the spleen where they induced local inflammatory gene and chemokine protein expression, and mobilized splenic-neutrophils to peripheral blood. Using CRISPR/Cas9 genome editing, we generated VCAM-1-deficient EC-EVs and showed that its deletion removed the ability of EC-EVs to provoke the mobilization of neutrophils. Furthermore, inhibition of miRNA-126 in vivo reduced myocardial infarction size in a mouse model.
Conclusions: Our findings show a novel EV-dependent mechanism for the rapid mobilization of neutrophils to peripheral blood from a splenic reserve and establish a proof of concept for functional manipulation of EV-communications through genetic alteration of parent cells.
Keywords: Exosome; Myocardial infarction; Programming; Spleen.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures




Comment in
-
Extracellular vesicles selectively mobilize splenic neutrophils.Cardiovasc Res. 2023 Mar 17;119(1):1-2. doi: 10.1093/cvr/cvad015. Cardiovasc Res. 2023. PMID: 36691968 No abstract available.
References
-
- Guo T-M, Cheng B, Ke L, Guan S-M, Qi B-L, Li W-Z, Yang B.. Prognostic value of neutrophil to lymphocyte ratio for in-hospital mortality in elderly patients with acute myocardial infarction. Curr Med Sci 2018;38:354–359. - PubMed
-
- Sreejit G, Abdel-Latif A, Athmanathan B, Annabathula R, Dhyani A, Noothi SK, Quaife-Ryan GA, Al-Sharea A, Pernes G, Dragoljevic D, Lal H, Schroder K, Hanaoka BY, Raman C, Grant MB, Hudson JE, Smyth SS, Porrello ER, Murphy AJ, Nagareddy PR.. Neutrophil-derived S100A8/A9 amplify granulopoiesis after myocardial infarction. Circulation 2020;141:1080–1094. - PMC - PubMed
-
- Chia S, Nagurney JT, Brown DFM, Raffel OC, Bamberg F, Senatore F, Wackers FJT, Jang I-K.. Association of leukocyte and neutrophil counts with infarct size, left ventricular function and outcomes after percutaneous coronary intervention for ST-elevation myocardial infarction. Am J Cardiol 2009;103:333–337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

