Efficacy, Safety, Pharmacokinetics, and Microbiome Changes of Ibezapolstat in Adults with Clostridioides difficile Infection: A Phase 2a Multicenter Clinical Trial
- PMID: 35134880
- PMCID: PMC9525077
- DOI: 10.1093/cid/ciac096
Efficacy, Safety, Pharmacokinetics, and Microbiome Changes of Ibezapolstat in Adults with Clostridioides difficile Infection: A Phase 2a Multicenter Clinical Trial
Abstract
Background: This study was the first human validation of the gram-positive bacterial DNA polymerase IIIC target in patients with Clostridioides difficile infection. The primary objectives were to assess clinical cure rates and adverse events (AEs). Secondary objectives were to evaluate plasma/fecal pharmacokinetics, microbiologic eradication, microbiome and bile acid effects, and sustained clinical cure (SCC) with ibezapolstat.
Methods: This single-arm, open-label, phase 2a study enrolled adults with C. difficile infection at 4 US centers. Patients received ibezapolstat 450 mg orally every 12 hours for 10 days and followed for an additional 28 days to assess study objectives.
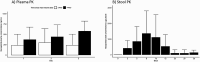
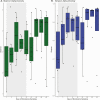
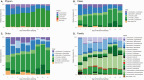
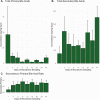
Results: Ten patients with a mean (standard deviation [SD]) age of 49 [15] years were enrolled. Seven AEs were reported classified as mild-moderate. Plasma levels of ibezapolstat ranged from 233 to 578 ng/mL while mean (SD) fecal levels were 416 (494) µg/g stool by treatment day 3 and >1000 µg/g stool by days 8-10. A rapid increase in alpha diversity in the fecal microbiome was noted after starting ibezapolstat therapy, which was maintained after completion of therapy. A proportional decrease in Bacteroidetes phylum was observed (mean change [SD], -10.0% [4.8%]; P = .04) with a concomitantly increased proportion of Firmicutes phylum (+14.7% [5.4%]; P = .009). Compared with baseline, total primary bile acids decreased by a mean (SD) of 40.1 (9.6) ng/mg stool during therapy (P < .001) and 40.5 (14.1) ng/mg stool after completion of therapy (P = .007). Rates of both initial clinical cure and SCC at 28 days were 100% (10 of 10 patients).
Conclusions: In this phase 2a study, 10 of 10 patients achieved SCC, demonstrated favorable pharmacokinetics, minimal AEs, and beneficial microbiome and bile acids results. These results support continued clinical development.
Trial registration: ClinicalTrials.gov NCT04247542.
Keywords: Clostridioides difficile; DNA polymerase IIIC; Enterocolitis; Female; Male; humans.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. K. W. G. received research grant support from Acurx Pharmaceuticals, Paratek, and Summit Pharmaceuticals. M. H. S. is a shareholder and paid consultant for Acurx Pharmaceuticals and received consulting fees from Summit, Paratek, Tetraphase, and Seres. M. H. S. is a shareholder of and paid consultant to Acurx Pharmaceuticals, Inc. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Functional and Metagenomic Evaluation of Ibezapolstat for Early Evaluation of Anti-Recurrence Effects in Clostridioides difficile Infection.Antimicrob Agents Chemother. 2022 Aug 16;66(8):e0224421. doi: 10.1128/aac.02244-21. Epub 2022 Jul 6. Antimicrob Agents Chemother. 2022. PMID: 35862742 Free PMC article.
-
A randomized, double-blind, placebo-controlled, single and multiple ascending dose Phase 1 study to determine the safety, pharmacokinetics and food and faecal microbiome effects of ibezapolstat administered orally to healthy subjects.J Antimicrob Chemother. 2020 Dec 1;75(12):3635-3643. doi: 10.1093/jac/dkaa364. J Antimicrob Chemother. 2020. PMID: 32892222 Free PMC article. Clinical Trial.
-
In vitro activity of the novel antibacterial agent ibezapolstat (ACX-362E) against Clostridioides difficile.J Antimicrob Chemother. 2020 Aug 1;75(8):2149-2155. doi: 10.1093/jac/dkaa134. J Antimicrob Chemother. 2020. PMID: 32285102
-
Review and Commentary on the Importance of Bile Acids in the Life Cycle of Clostridioides difficile in Children and Adults.J Pediatric Infect Dis Soc. 2021 May 28;10(5):659-664. doi: 10.1093/jpids/piaa150. J Pediatric Infect Dis Soc. 2021. PMID: 33626138 Review.
-
Ridinilazole: a novel, narrow-spectrum antimicrobial agent targeting Clostridium (Clostridioides) difficile.Lett Appl Microbiol. 2022 Sep;75(3):526-536. doi: 10.1111/lam.13664. Epub 2022 Feb 11. Lett Appl Microbiol. 2022. PMID: 35119124 Free PMC article. Review.
Cited by
-
European Practice for CDI Treatment.Adv Exp Med Biol. 2024;1435:57-84. doi: 10.1007/978-3-031-42108-2_4. Adv Exp Med Biol. 2024. PMID: 38175471
-
Clostridium difficile infection in pediatric patients (Review).Biomed Rep. 2023 Dec 8;20(2):18. doi: 10.3892/br.2023.1706. eCollection 2024 Feb. Biomed Rep. 2023. PMID: 38169799 Free PMC article. Review.
-
Antibiotics in the clinical pipeline as of December 2022.J Antibiot (Tokyo). 2023 Aug;76(8):431-473. doi: 10.1038/s41429-023-00629-8. Epub 2023 Jun 8. J Antibiot (Tokyo). 2023. PMID: 37291465 Free PMC article. Review.
-
Pre-Steady-State Kinetic Characterization of an Antibiotic-Resistant Mutant of Staphylococcus aureus DNA Polymerase PolC.Antimicrob Agents Chemother. 2023 Jun 15;67(6):e0157122. doi: 10.1128/aac.01571-22. Epub 2023 May 24. Antimicrob Agents Chemother. 2023. PMID: 37222615 Free PMC article.
-
The microbiome-restorative potential of ibezapolstat for the treatment of Clostridioides difficile infection is predicted through variant PolC-type DNA polymerase III in Lachnospiraceae and Oscillospiraceae.Antimicrob Agents Chemother. 2025 Apr 2;69(4):e0167924. doi: 10.1128/aac.01679-24. Epub 2025 Feb 21. Antimicrob Agents Chemother. 2025. PMID: 39982073 Free PMC article.
References
-
- Johnson S, Lavergne V, Skinner AM, et al. . Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis 2021; 73:755–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

