Purkinje network and myocardial substrate at the onset of human ventricular fibrillation: implications for catheter ablation
- PMID: 35134898
- PMCID: PMC8934691
- DOI: 10.1093/eurheartj/ehab893
Purkinje network and myocardial substrate at the onset of human ventricular fibrillation: implications for catheter ablation
Abstract
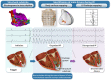
Aims: Mapping data of human ventricular fibrillation (VF) are limited. We performed detailed mapping of the activities underlying the onset of VF and targeted ablation in patients with structural cardiac abnormalities.
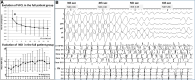
Methods and results: We evaluated 54 patients (50 ± 16 years) with VF in the setting of ischaemic (n = 15), hypertrophic (n = 8) or dilated cardiomyopathy (n = 12), or Brugada syndrome (n = 19). Ventricular fibrillation was mapped using body-surface mapping to identify driver (reentrant and focal) areas and invasive Purkinje mapping. Purkinje drivers were defined as Purkinje activities faster than the local ventricular rate. Structural substrate was delineated by electrogram criteria and by imaging. Catheter ablation was performed in 41 patients with recurrent VF. Sixty-one episodes of spontaneous (n = 10) or induced (n = 51) VF were mapped. Ventricular fibrillation was organized for the initial 5.0 ± 3.4 s, exhibiting large wavefronts with similar cycle lengths (CLs) across both ventricles (197 ± 23 vs. 196 ± 22 ms, P = 0.9). Most drivers (81%) originated from areas associated with the structural substrate. The Purkinje system was implicated as a trigger or driver in 43% of patients with cardiomyopathy. The transition to disorganized VF was associated with the acceleration of initial reentrant activities (CL shortening from 187 ± 17 to 175 ± 20 ms, P < 0.001), then spatial dissemination of drivers. Purkinje and substrate ablation resulted in the reduction of VF recurrences from a pre-procedural median of seven episodes [interquartile range (IQR) 4-16] to 0 episode (IQR 0-2) (P < 0.001) at 56 ± 30 months.
Conclusions: The onset of human VF is sustained by activities originating from Purkinje and structural substrate, before spreading throughout the ventricles to establish disorganized VF. Targeted ablation results in effective reduction of VF burden.
Key question: The initial phase of human ventricular fibrillation (VF) is critical as it involves the primary activities leading to sustained VF and arrhythmic sudden death. The origin of such activities is unknown.
Key finding: Body-surface mapping shows that most drivers (≈80%) during the initial VF phase originate from electrophysiologically defined structural substrates. Repetitive Purkinje activities can be elicited by programmed stimulation and are implicated as drivers in 37% of cardiomyopathy patients.
Take-home message: The onset of human VF is mostly associated with activities from the Purkinje network and structural substrate, before spreading throughout the ventricles to establish sustained VF. Targeted ablation reduces or eliminates VF recurrence.
Keywords: Ablation; Brugada syndrome; Cardiomyopathy; Purkinje system; Sudden cardiac death; Ventricular fibrillation.
© The Author(s) 2022. Published by Oxford University Press on behalf of European Society of Cardiology.
Figures





Comment in
-
Ventricular fibrillation: combined myocardial substrate and Purkinje ablation.Eur Heart J. 2022 Mar 21;43(12):1248-1250. doi: 10.1093/eurheartj/ehab912. Eur Heart J. 2022. PMID: 35139150 No abstract available.
References
-
- Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2015;36:2793–2867. - PubMed
-
- Weiss JN, Chen P-S, Qu Z, Karagueuzian HS, Garfinkel A. Ventricular fibrillation: how do we stop the waves from breaking? Circ Res 2000;87:1103–1107. - PubMed
-
- Valderrábano M, Chen P-S, Lin S-F. Spatial distribution of phase singularities in ventricular fibrillation. Circulation 2003;108:354–359. - PubMed
-
- Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, et al. Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 2006;114:536–542. - PubMed

