Biomarkers of Exposure and Biomarkers of Potential Harm in Adult Smokers Who Switch to e-Vapor Products Relative to Cigarette Smoking in a 24-week, Randomized, Clinical Trial
- PMID: 35134961
- PMCID: PMC9199942
- DOI: 10.1093/ntr/ntac029
Biomarkers of Exposure and Biomarkers of Potential Harm in Adult Smokers Who Switch to e-Vapor Products Relative to Cigarette Smoking in a 24-week, Randomized, Clinical Trial
Abstract
Introduction: Long-term health effects of e-vapor products (EVPs) are not well-established. We compared biomarkers of exposure (BoE) to select harmful and potentially harmful constituents and biomarkers of potential harm (BoPH) in adult smokers who switched to EVPs versus continued smoking for 24 weeks.
Methods: Adult smokers (n = 450, >10 cigarettes per day for ≥10 years) were randomly assigned to continue smoking (control) or switch to one of two cartridge-based EVPs (test 1: classic; test 2: menthol, 4% nicotine). BoE and BoPH were measured at baseline and 12 weeks. The results presented here are from a subset of 150 control and EVP subjects (switchers with exhaled carbon monoxide <8 ppm and <10% baseline cigarettes per day) followed for 24 total weeks.
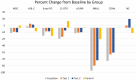
Results: Total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and carboxyhemoglobin were significantly reduced (p < .0001) in tests 1 and 2 at 24 weeks. Urinary nicotine equivalents were not statistically significantly different between the control and EVP groups. At week 24, statistically significant reductions (p < .05) were observed for white blood cell counts, 11-dehydrothromboxane β2, and sICAM in both test groups, and there were several significant changes in measures of pulmonary function. High-density lipoprotein cholesterol and 8-epi-prostaglandin-F2α were directionally favorable in both EVP groups versus control.
Conclusions: We demonstrate that significant reductions of selected harmful and potentially harmful constituents in EVP aerosol results in significant reductions in BoEs and favorable changes in BoPHs after switching to EVPs for 24 weeks. These changes approached those reported for smoking cessation, suggesting that switching to exclusive use of the EVPs may be less harmful than continuing smoking.
Implications: Cigarette smoking causes serious diseases. Switching from cigarettes to a noncombustible product is a potential harm reduction pathway for adult smokers unable or unwilling to quit. Long-term health effects of e-vapor products (EVPs) compared with continued smoking have not been extensively studied. We present biomarker of exposure evidence on select harmful and potentially harmful constituents and biomarkers of potential harm related to inflammation and oxidative stress in adult smokers switching to two EVPs. This study demonstrates significant reductions in biomarkers of exposure (except for nicotine) accompanied with favorable changes in various biomarkers of potential harm, including pulmonary function. The totality of evidence suggests that exclusive EVP use may present lower health risks compared with smoking cigarettes.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Society for Research on Nicotine and Tobacco.
Figures
References
-
- Gottlieb S, Zeller MA. Nicotine-focused framework for public health. N Engl J Med. 2017;377(12):1111–1114. - PubMed
-
- Wagner KA, Flora JW, Melvin MS, et al. . An evaluation of electronic cigarette formulations and aerosols for harmful and potentially harmful constituents (HPHCs) typically derived from combustion. Regul Toxicol Pharmacol. 2018;95:153–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

