The effects of phylogeny, habitat and host characteristics on the thermal sensitivity of helminth development
- PMID: 35135354
- PMCID: PMC8825990
- DOI: 10.1098/rspb.2021.1878
The effects of phylogeny, habitat and host characteristics on the thermal sensitivity of helminth development
Abstract
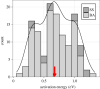
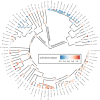
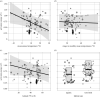
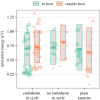
Helminth parasites are part of almost every ecosystem, with more than 300 000 species worldwide. Helminth infection dynamics are expected to be altered by climate change, but predicting future changes is difficult owing to lacking thermal sensitivity data for greater than 99.9% of helminth species. Here, we compiled the largest dataset to date on helminth temperature sensitivities and used the Metabolic Theory of Ecology to estimate activation energies (AEs) for parasite developmental rates. The median AE for 129 thermal performance curves was 0.67, similar to non-parasitic animals. Although exceptions existed, related species tended to have similar thermal sensitivities, suggesting some helminth taxa are inherently more affected by rising temperatures than others. Developmental rates were more temperature-sensitive for species from colder habitats than those from warmer habitats, and more temperature sensitive for species in terrestrial than aquatic habitats. AEs did not depend on whether helminth life stages were free-living or within hosts, whether the species infected plants or animals, or whether the species had an endotherm host in its life cycle. The phylogenetic conservatism of AE may facilitate predicting how temperature change affects the development of helminth species for which empirical data are lacking or difficult to obtain.
Keywords: activation energy; climate change; metabolic theory; parasite.
Figures
References
-
- Lafferty K. 2008. Effects of disease on community interactions and food web structure. In Infectious disease ecology: effects of ecosystems on disease and of disease on ecosystems (eds Ostfeld RS, Keesing F, Eviner VT), pp. 205-222. Princeton, NJ: Princeton University Press.
-
- Dobson AP, Hudson PJ. 1992. Regulation and stability of a free-living host-parasite system: Trichostrongylus tenuis in red grouse. II. Population models. J. Anim. Ecol. 61, 487-498. (10.2307/5339) - DOI
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
