Potassium responses to sodium zirconium cyclosilicate in hyperkalemic hemodialysis patients: post-hoc analysis of DIALIZE
- PMID: 35135481
- PMCID: PMC8826669
- DOI: 10.1186/s12882-021-02569-7
Potassium responses to sodium zirconium cyclosilicate in hyperkalemic hemodialysis patients: post-hoc analysis of DIALIZE
Abstract
Background: Sodium zirconium cyclosilicate (SZC) is an effective and well-tolerated treatment for hyperkalemia in maintenance hemodialysis patients. In post-hoc analyses of the phase 3b DIALIZE study, we examined the spectrum of potassium responses to SZC.
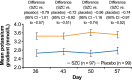
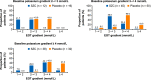
Methods: Post-hoc analyses with SZC and placebo included: the number of long interdialytic interval (LIDI) visits during the 4-week evaluation period where patients attained pre-dialysis serum potassium (sK+) concentrations of 4.0-5.0 and 4.0-5.5 mmol/L; potassium gradient (the difference between pre-dialysis sK+ and dialysate potassium) at days 36, 43, 50, and 57, and change from baseline to the end of treatment (EOT) using categories of potassium gradient (1 to < 2, 2 to < 3, 3 to < 4, and ≥ 4 mmol/L).
Results: A greater proportion of patients achieved the ranges of pre-dialysis sK+ concentration with SZC versus placebo for ≥1, ≥ 2, ≥ 3, and 4 LIDI visits over 4 weeks; 23.7 and 48.5% of patients in the SZC group achieved pre-dialysis sK+ concentrations of 4.0-5.0 and 4.0-5.5 mmol/L, respectively, at all 4 LIDI visits. Baseline mean potassium gradient was similar with SZC and placebo. At day 57, mean (standard deviation) potassium gradient was 2.78 (0.08) mmol/L with SZC and 3.52 (0.08) mmol/L with placebo; mean difference (95% confidence interval) was - 0.74 mmol/L (- 0.97 to - 0.52). A greater reduction in potassium gradient category from baseline towards lower-risk categories at EOT was observed with SZC versus placebo.
Conclusions: These analyses expand our knowledge of the spectrum of potassium responses with SZC in hyperkalemic hemodialysis patients.
Trial registration: NCT03303521 .
Keywords: Chronic kidney disease; Hemodialysis; Hyperkalemia; Potassium; Sodium zirconium cyclosilicate.
© 2022. The Author(s).
Conflict of interest statement
SF received research support and consulting fees from AstraZeneca. MFord received travel support from Amgen and AstraZeneca, and is an advisory board member for AstraZeneca. MFukagawa received consulting fees and lectures fees from AstraZeneca Japan. KM is an academic grant holder and advisory board member for AstraZeneca. AR received research or travel support from and/or is a speaker, consultant, or advisory board member for AstraZeneca, Relypsa, Fresenius Medical Care, Sanofi, Kadmon, AMAG, Otsuka, Genzyme, GSK, Omerus, Janssen, Reata Pharmaceuticals, Ironwood, and Amgen. BS received research grants, lecture fees and/or consulting fees from AstraZeneca, Akebia, Reata Pharmaceuticals, and Fresenius Medical Care. KS received research support from AstraZeneca. KV received research support from AstraZeneca. VL, AAS, and NG are employees of AstraZeneca. SB has given lectures and participated in an advisory board for AstraZeneca, has given lectures sponsored by Vifor Pharma, and has received travel support from AstraZeneca and Vifor Pharma.
Figures

Similar articles
-
DIALIZE China: A Phase IIIb, Randomized, Placebo-Controlled Study to Reduce Predialysis Hyperkalemia With Sodium Zirconium Cyclosilicate in Chinese Patients.Clin Ther. 2023 Jul;45(7):633-642. doi: 10.1016/j.clinthera.2023.04.014. Epub 2023 Jun 27. Clin Ther. 2023. PMID: 37385905 Clinical Trial.
-
A Phase 3b, Randomized, Double-Blind, Placebo-Controlled Study of Sodium Zirconium Cyclosilicate for Reducing the Incidence of Predialysis Hyperkalemia.J Am Soc Nephrol. 2019 Sep;30(9):1723-1733. doi: 10.1681/ASN.2019050450. Epub 2019 Jun 14. J Am Soc Nephrol. 2019. PMID: 31201218 Free PMC article. Clinical Trial.
-
Effects of dialysate potassium concentration of 3.0 mmol/l with sodium zirconium cyclosilicate on dialysis-free days versus dialysate potassium concentration of 2.0 mmol/l alone on rates of cardiac arrhythmias in hemodialysis patients with hyperkalemia.Kidney Int. 2025 Jan;107(1):169-179. doi: 10.1016/j.kint.2024.10.010. Epub 2024 Oct 26. Kidney Int. 2025. PMID: 39490411 Clinical Trial.
-
Effects and Safety of a Novel Oral Potassium-Lowering Drug-Sodium Zirconium Cyclosilicate for the Treatment of Hyperkalemia: a Systematic Review and Meta-Analysis.Cardiovasc Drugs Ther. 2021 Oct;35(5):1057-1066. doi: 10.1007/s10557-020-07134-2. Epub 2021 Jan 18. Cardiovasc Drugs Ther. 2021. PMID: 33459923 Free PMC article.
-
An evaluation of sodium zirconium cyclosilicate as a treatment option for hyperkalemia.Expert Opin Pharmacother. 2021 Jan;22(1):19-28. doi: 10.1080/14656566.2020.1810234. Epub 2020 Sep 7. Expert Opin Pharmacother. 2021. PMID: 32892634 Review.
Cited by
-
Recommendations for the management of hyperkalemia in patients receiving renin-angiotensin-aldosterone system inhibitors.Intern Emerg Med. 2024 Mar;19(2):295-306. doi: 10.1007/s11739-023-03427-0. Epub 2023 Sep 29. Intern Emerg Med. 2024. PMID: 37775712 Free PMC article. Review.
-
Hyperkalemia burden and treatment patterns in Chinese patients on hemodialysis: final analysis of a prospective multicenter cohort study (PRECEDE-K).Ren Fail. 2024 Dec;46(2):2384585. doi: 10.1080/0886022X.2024.2384585. Epub 2024 Sep 9. Ren Fail. 2024. PMID: 39252179 Free PMC article.
-
Hyperkalemia: Pharmacotherapies and Clinical Considerations.Cureus. 2024 Jan 26;16(1):e52994. doi: 10.7759/cureus.52994. eCollection 2024 Jan. Cureus. 2024. PMID: 38406030 Free PMC article. Review.
-
Hyperkalemia management: a multidisciplinary expert panel's perspective on the role of new potassium binders.Heart Fail Rev. 2025 Mar;30(2):271-286. doi: 10.1007/s10741-024-10461-3. Epub 2024 Nov 27. Heart Fail Rev. 2025. PMID: 39604607 Free PMC article. Review.
-
Hyperkalaemia prevalence and dialysis patterns in Chinese patients on haemodialysis: an interim analysis of a prospective cohort study (PRECEDE-K).BMC Nephrol. 2023 Aug 9;24(1):233. doi: 10.1186/s12882-023-03261-8. BMC Nephrol. 2023. PMID: 37559023 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

