CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis
- PMID: 35135557
- PMCID: PMC8822708
- DOI: 10.1186/s12987-021-00299-4
CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis
Abstract
Background: Multiple sclerosis (MS) is a complex, heterogenous disease characterized by inflammation, demyelination, and blood-brain barrier (BBB) permeability. Currently, active disease is determined by physician confirmed relapse or detection of contrast enhancing lesions via MRI indicative of BBB permeability. However, clinical confirmation of active disease can be cumbersome. As such, disease monitoring in MS could benefit from identification of an easily accessible biomarker of active disease. We believe extracellular vesicles (EV) isolated from plasma are excellent candidates to fulfill this need. Because of the critical role BBB permeability plays in MS pathogenesis and identification of active disease, we sought to identify EV originating from central nervous system (CNS) endothelial as biomarkers of active MS. Because endothelial cells secrete more EV when stimulated or injured, we hypothesized that circulating concentrations of CNS endothelial derived EV will be increased in MS patients with active disease.
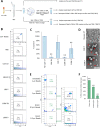
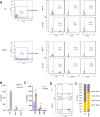
Methods: To test this, we developed a novel method to identify EV originating from CNS endothelial cells isolated from patient plasma using flow cytometry. Endothelial derived EV were identified by the absence of lymphocyte or platelet markers CD3 and CD41, respectively, and positive expression of pan-endothelial markers CD31, CD105, or CD144. To determine if endothelial derived EV originated from CNS endothelial cells, EV expressing CD31, CD105, or CD144 were evaluated for expression of the myelin and lymphocyte protein MAL, a protein specifically expressed by CNS endothelial cells compared to endothelial cells of peripheral organs.
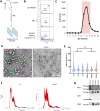
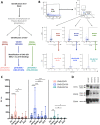
Results: Quality control experiments indicate that EV detected using our flow cytometry method are 0.2 to 1 micron in size. Flow cytometry analysis of EV isolated from 20 healthy controls, 16 relapsing-remitting MS (RRMS) patients with active disease not receiving disease modifying therapy, 14 RRMS patients with stable disease not receiving disease modifying therapy, 17 relapsing-RRMS patients with stable disease receiving natalizumab, and 14 RRMS patients with stable disease receiving ocrelizumab revealed a significant increase in the plasma concentration of CNS endothelial derived EV in patients with active disease compared to all other groups (p = 0.001).
Conclusions: For the first time, we have identified a method to identify CNS endothelial derived EV in circulation from human blood samples. Results from our pilot study indicate that increased levels of CNS endothelial derived EV may be a biomarker of BBB permeability and active disease in MS.
Keywords: Biomarker; Blood–brain barrier; Endothelial cells; Extracellular vesicles; Multiple sclerosis.
© 2022. The Author(s).
Conflict of interest statement
Antibody JL004 used in this publication is the subject of granted and pending patent applications.
Figures



Similar articles
-
Assessment of technical and clinical utility of a bead-based flow cytometry platform for multiparametric phenotyping of CNS-derived extracellular vesicles.Cell Commun Signal. 2023 Oct 6;21(1):276. doi: 10.1186/s12964-023-01308-9. Cell Commun Signal. 2023. PMID: 37803478 Free PMC article.
-
Proteomic profile of extracellular vesicles from plasma and CSF of multiple sclerosis patients reveals disease activity-associated EAAT2.J Neuroinflammation. 2024 Sep 2;21(1):217. doi: 10.1186/s12974-024-03148-x. J Neuroinflammation. 2024. PMID: 39223661 Free PMC article.
-
Extracellular Vesicle Marker Changes Associated With Disease Activity in Relapsing-Remitting Multiple Sclerosis.Neurol Neuroimmunol Neuroinflamm. 2025 Jul;12(4):e200404. doi: 10.1212/NXI.0000000000200404. Epub 2025 Apr 29. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 40300121 Free PMC article.
-
Extracellular vesicles: mediators and biomarkers of pathology along CNS barriers.Fluids Barriers CNS. 2018 Jul 1;15(1):19. doi: 10.1186/s12987-018-0104-7. Fluids Barriers CNS. 2018. PMID: 29960602 Free PMC article. Review.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
Assessment of technical and clinical utility of a bead-based flow cytometry platform for multiparametric phenotyping of CNS-derived extracellular vesicles.Cell Commun Signal. 2023 Oct 6;21(1):276. doi: 10.1186/s12964-023-01308-9. Cell Commun Signal. 2023. PMID: 37803478 Free PMC article.
-
Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy.Int J Mol Sci. 2025 Jul 26;26(15):7246. doi: 10.3390/ijms26157246. Int J Mol Sci. 2025. PMID: 40806377 Free PMC article. Review.
-
Extracellular Vesicles in Chronic Demyelinating Diseases: Prospects in Treatment and Diagnosis of Autoimmune Neurological Disorders.Life (Basel). 2022 Nov 21;12(11):1943. doi: 10.3390/life12111943. Life (Basel). 2022. PMID: 36431078 Free PMC article. Review.
-
Exosomes in inflammation and cancer: from bench to bedside applications.Mol Biomed. 2025 Jun 10;6(1):41. doi: 10.1186/s43556-025-00280-9. Mol Biomed. 2025. PMID: 40490663 Free PMC article. Review.
-
Emerging imaging and liquid biomarkers in multiple sclerosis.Eur J Immunol. 2023 Aug;53(8):e2250228. doi: 10.1002/eji.202250228. Epub 2023 May 28. Eur J Immunol. 2023. PMID: 37194443 Free PMC article. Review.
References
-
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. - PubMed
-
- Andaloussi SE, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. - PubMed
-
- van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. - PubMed
-
- Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

