Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels
- PMID: 35135594
- PMCID: PMC8822809
- DOI: 10.1186/s13287-022-02717-2
Synergistic regenerative therapy of thin endometrium by human placenta-derived mesenchymal stem cells encapsulated within hyaluronic acid hydrogels
Abstract
Background: Thin endometrium is a primary cause of defective endometrial receptivity, resulting in infertility or recurrent miscarriage. Much effort has been devoted toward regenerating thin endometrium by stem cell-based therapies. The human placenta-derived mesenchymal stem cells (HP-MSCs) are emerging alternative sources of MSCs with various advantages. To maximize their retention inside the uterus, we loaded HP-MSCs with cross-linked hyaluronic acid hydrogel (HA hydrogel) to investigate their therapeutic efficacy and possible underlying mechanisms.
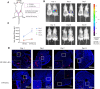
Methods: Ethanol was injected into the mice uterus to establish the endometrium-injured model. The retention time of HP-MSCs and HA hydrogel was detected by in vivo imaging, while the distribution of HP-MSCs was detected by immunofluorescence staining. Functional restoration of the uterus was assessed by testing embryo implantation rates. The endometrial morphological alteration was observed by H&E staining, Masson staining, and immunohistochemistry. In vitro studies were further conducted using EdU, transwell, tube formation, and western blot assays.
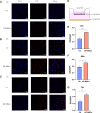
Results: Instilled HP-MSCs with HA hydrogel (HP-MSCs-HA) exhibited a prolonged retention time in mouse uteri than normal HP-MSCs. In vivo studies showed that the HP-MSCs-HA could significantly increase the gland number and endometrial thickness (P < 0.001, P < 0.05), decrease fibrous area (P < 0.0001), and promote the proliferation and angiogenesis of endometrial cells (as indicated by Ki67 and VEGF, P < 0.05, P < 0.05, respectively) in mice injured endometrium. HP-MSCs-HA could also significantly improve the embryo implantation rate (P < 0.01) compared with the ethanol group. Further mechanistic study showed the paracrine effects of HP-MSCs. They could not only promote the proliferation and migration of human endometrial stromal cells via the JNK/Erk1/2-Stat3-VEGF pathway but also facilitate the proliferation of glandular cells via Jak2-Stat5 and c-Fos-VEGF pathway. In turn, the increased VEGF in the endometrium promoted the angiogenesis of endothelial cells.
Conclusion: Our study suggested the potential therapeutic effects and the underlying mechanisms of HP-MSCs-HA on treating thin endometrium. HA hydrogel could be a preferable delivery method for HP-MSCs, and the strategy represents a promising therapeutic approach against endometrial injury in clinical settings.
Keywords: Endometrial repair; Human placenta-derived mesenchymal stem cells; Hyaluronic acid hydrogels; Regeneration mechanisms; Thin endometrium.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Frydman R, Chaouat G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod. 2002;17(1):213–218. - PubMed
-
- Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17(5):555–569. - PubMed
-
- Cenksoy P, Ficicioglu C, Yıldırım G, Yesiladali M. Hysteroscopic findings in women with recurrent IVF failures and the effect of correction of hysteroscopic findings on subsequent pregnancy rates. Arch Gynecol Obstet. 2013;287(2):357–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

