Evaluation of the effect of maternally derived antibody on response to MMR vaccine in Thai infants
- PMID: 35135700
- PMCID: PMC8884255
- DOI: 10.1016/j.vaccine.2022.01.049
Evaluation of the effect of maternally derived antibody on response to MMR vaccine in Thai infants
Abstract
Background: Although the number of measles cases declined globally in response to anti-measles immunisation campaigns, measles has re-emerged. A review of current vaccination policies is required to improve measles elimination strategies.
Methods: A pseudotype-based virus neutralisation assay (PVNA) was used to measure neutralising antibody titres in serum samples collected from Thai infants at six timepoints before and after two-doses of MMR (1&2) vaccination (ClinicalTrials.gov no. NCT02408926). Vesicular stomatitis virus (VSV) luciferase pseudotypes bearing the haemaglutinin (H) and fusion (F) glycoproteins of measles virus (MeV) were prepared. Serial dilutions of serum samples were incubated with VSV (MeV) pseudotypes and plated onto HEK293-human SLAM1 cells; the neutralising antibody titre was defined as the dilution resulting in 90% reduction in luciferase activity.
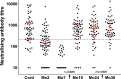
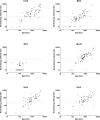
Results: Neutralising antibody titres in infants born with high levels of maternal immunity (H group) persisted at the time of the first MMR vaccination, and those infants did not respond effectively by developing protective titres. In contrast, infants with lower maternal immunity (L group) developed protective titres of antibody following vaccination. Responses to the second MMR vaccination were significantly higher (P = 0.0171, Wilcoxon signed-rank test) in the H group. The observed correlation between anti-MeV IgG level and neutralising antibody titre in Thai infants indicates the possibility of using rapid IgG testing as a surrogate measure for neutralising activity to define clinical protection levels within populations.
Conclusion: These results demonstrate that varying the timing of the first MMR immunisation according to the level of acquired maternal immunity could increase vaccination immunogenicity and hence accelerate measles eradication.
Keywords: Childhood vaccination; Maternal immunity; Measles-mumps-rubella (MMR); Neutralising antibody; Pseudotype-based virus neutralisation assay.
Crown Copyright © 2022. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Patel M.K., Gacic-Dobo M., Strebel P.M., Dabbagh A., Mulders M.N., Okwo-Bele J.-M., et al. Progress toward regional measles elimination - worldwide, 2000–2015. MMWR Morb Mortal Wkly Rep. 2016;65(44):1228–1233. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

