Lactate Rewires Lipid Metabolism and Sustains a Metabolic-Epigenetic Axis in Prostate Cancer
- PMID: 35135811
- PMCID: PMC7612586
- DOI: 10.1158/0008-5472.CAN-21-0914
Lactate Rewires Lipid Metabolism and Sustains a Metabolic-Epigenetic Axis in Prostate Cancer
Abstract
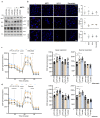
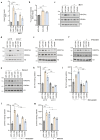
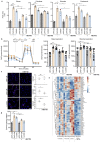
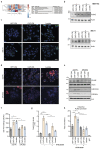
Lactate is an abundant oncometabolite in the tumor environment. In prostate cancer, cancer-associated fibroblasts (CAF) are major contributors of secreted lactate, which can be taken up by cancer cells to sustain mitochondrial metabolism. However, how lactate impacts transcriptional regulation in tumors has yet to be fully elucidated. Here, we describe a mechanism by which CAF-secreted lactate is able to increase the expression of genes involved in lipid metabolism in prostate cancer cells. This regulation enhanced intracellular lipid accumulation in lipid droplets (LD) and provided acetyl moieties for histone acetylation, establishing a regulatory loop between metabolites and epigenetic modification. Inhibition of this loop by targeting the bromodomain and extraterminal protein family of histone acetylation readers suppressed the expression of perilipin 2 (PLIN2), a crucial component of LDs, disrupting lactate-dependent lipid metabolic rewiring. Inhibition of this CAF-induced metabolic-epigenetic regulatory loop in vivo reduced growth and metastasis of prostate cancer cells, demonstrating its translational relevance as a therapeutic target in prostate cancer. Clinically, PLIN2 expression was elevated in tumors with a higher Gleason grade and in castration-resistant prostate cancer compared with primary prostate cancer. Overall, these findings show that lactate has both a metabolic and an epigenetic role in promoting prostate cancer progression.
Significance: This work shows that stromal-derived lactate induces accumulation of lipid droplets, stimulates epigenetic rewiring, and fosters metastatic potential in prostate cancer.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfør K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–21. - PubMed
-
- Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, et al. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:349–53. - PubMed
-
- Doyen J, Trastour C, Ettore F, Peyrottes I, Toussant N, Gal J, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun. 2014;451:54–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

