Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements
- PMID: 35135829
- PMCID: PMC9394398
- DOI: 10.1158/2159-8290.CD-21-1119
Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements
Abstract
NRG1 rearrangements are recurrent oncogenic drivers in solid tumors. NRG1 binds to HER3, leading to heterodimerization with other HER/ERBB kinases, increased downstream signaling, and tumorigenesis. Targeting ERBBs, therefore, represents a therapeutic strategy for these cancers. We investigated zenocutuzumab (Zeno; MCLA-128), an antibody-dependent cellular cytotoxicity-enhanced anti-HER2xHER3 bispecific antibody, in NRG1 fusion-positive isogenic and patient-derived cell lines and xenograft models. Zeno inhibited HER3 and AKT phosphorylation, induced expression of apoptosis markers, and inhibited growth. Three patients with chemotherapy-resistant NRG1 fusion-positive metastatic cancer were treated with Zeno. Two patients with ATP1B1-NRG1-positive pancreatic cancer achieved rapid symptomatic, biomarker, and radiographic responses and remained on treatment for over 12 months. A patient with CD74-NRG1-positive non-small cell lung cancer who had progressed on six prior lines of systemic therapy, including afatinib, responded rapidly to treatment with a partial response. Targeting HER2 and HER3 simultaneously with Zeno is a novel therapeutic paradigm for patients with NRG1 fusion-positive cancers.
Significance: NRG1 rearrangements encode chimeric ligands that activate the ERBB receptor tyrosine kinase family. Here we show that targeting HER2 and HER3 simultaneously with the bispecific antibody Zeno leads to durable clinical responses in patients with NRG1 fusion-positive cancers and is thus an effective therapeutic strategy. This article is highlighted in the In This Issue feature, p. 1171.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

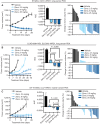
![Figure 4. Clinical responses to Zeno. A, Clinical course of a 50-year-old man with ATP1B1–NRG1 fusion–positive PDAC treated with Zeno (top) including tumor volume and CA 19-9 levels during Zeno treatment (bottom). Best overall response is indicated for each therapy, including progressive clinical disease (PD) and partial response (PR) as defined by RECIST v1.1. B, Representative tumor imaging of this patient's liver metastases at baseline and eight weeks into treatment with Zeno. C, Clinical course of a 34-year-old man with ATP1B1–NRG1 fusion–positive PDAC treated with Zeno (top) including tumor volume and CA 19-9 levels during Zeno treatment (bottom). Best overall response is indicated for Zeno [stable disease (SD) as defined by RECIST v1.1]. D, Representative tumor imaging from this patient showing a CT scan of the pancreas performed at baseline and seven weeks into treatment with Zeno, and a PET scan 10 weeks into treatment showing non–FDG-avid liver metastases (no baseline available). E, Clinical course of a 52-year-old man with CD74–NRG1 fusion–positive NSCLC treated with Zeno after six prior lines of systemic therapy and multiple courses of radiation. Best overall response is labeled for each therapy, including clinical PD/SD, and PR as defined by RECIST v1.1. F, Tumor shrinkage in this patient depicted graphically (left) and by representative tumor imaging (right) performed at baseline and 16 weeks into Zeno treatment. Abbreviations: cape, capecitabine; carbo, carboplatin; gem, gemcitabine; MDS-EB1, myelodysplastic syndrome with excess blasts-1; pem, pemetrexed; PORT, postoperative radiotherapy; vin, vinorelbine.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/c537/9394398/6df9296bc1ac/1233fig4.gif)
Comment in
- Cancer Discov. 12:1171.
- Cancer Discov. 12:1171.
References
-
- Ptakova N, Martinek P, Holubec L, Janovsky V, Vancurova J, Grossmann P, et al. Identification of tumors with NRG1 rearrangement, including a novel putative pathogenic UNC5D-NRG1 gene fusion in prostate cancer by data-drilling a de-identified tumor database. Genes Chromosomes Cancer 2021;60:474–81. - PubMed
-
- Huang HE, Chin SF, Ginestier C, Bardou VJ, Adelaide J, Iyer NG, et al. A recurrent chromosome breakpoint in breast cancer at the NRG1/neuregulin 1/heregulin gene. Cancer Res 2004;64:6840–4. - PubMed
-
- Fernandez-Cuesta L, Plenker D, Osada H, Sun R, Menon R, Leenders F, et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov 2014;4:415–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

