Morphology and Dendrite-Specific Synaptic Properties of Midbrain Neurons Shape Multimodal Integration
- PMID: 35135851
- PMCID: PMC8973417
- DOI: 10.1523/JNEUROSCI.1695-21.2022
Morphology and Dendrite-Specific Synaptic Properties of Midbrain Neurons Shape Multimodal Integration
Abstract
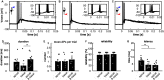
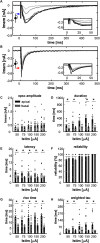
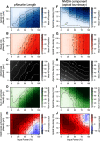
Multimodal integration facilitates object recognition and response to sensory cues. This depends on spatiotemporal coincidence of sensory information, recruitment of NMDA-type glutamate receptors and inhibitory feedback. Shepherd's crook neurons (SCNs) in the avian optic tectum (TeO) are an ideal model for studying cellular mechanism of multimodal integration. They receive different sensory modalities through spatially segregated dendrites, are important for stimulus selection and have an axon-carrying dendrite (AcD). We performed whole-cell patch-clamp experiments in chicken midbrain slices of both sexes. We emulated visual and auditory input in vitro by stimulating presynaptic afferents electrically. Simultaneous stimulation enhanced responses inversely depending on stimulation amplitude demonstrating the principle of inverse effectiveness. Contribution of NMDA-type glutamate receptors prolonged postsynaptic events for visual inputs only, causing a strong modality-specific difference in synaptic efficacy. We designed a multicompartment model to study the effect of morphological and physiological parameters on multimodal integration by varying the distance between soma and axonal origin and the amount of NMDA receptor (NMDAR) contribution. These parameters changed the preference of the model for one input channel and adjusted the range of input rates at which multimodal enhancement occurred on naturalistic stimulation. Thus, the unique morphology and synaptic features of SCNs shape the integration of input at different dendrites and generates an enhanced multimodal response.SIGNIFICANCE STATEMENT Multimodal integration improves perception and responses to objects. The underlying cellular mechanism depends on a balance between excitation and inhibition, and NMDA-type glutamate receptors that are involved in the multiplicative nature of enhancement following the principle of inverse effectiveness. Based on a detailed analysis of an identified multimodal cell type in the vertebrate midbrain, we studied the influence of cellular morphology and unimodal synaptic properties on multimodal integration. We can show that the combination of cellular morphology and modality-specific synaptic properties including NMDA receptor (NMDAR) contribution is optimal for nonlinear, multimodal enhancement and determines the dynamic response range of the integrating neuron. Our findings mechanistically explain how synaptic properties and cellular morphology of a midbrain neuron contribute to multimodal enhancement.
Keywords: NMDA; dendrite; midbrain; morphology; multimodal; tectum.
Copyright © 2022 the authors.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
