Nitric oxide stimulates type IV MSHA pilus retraction in Vibrio cholerae via activation of the phosphodiesterase CdpA
- PMID: 35135874
- PMCID: PMC8851539
- DOI: 10.1073/pnas.2108349119
Nitric oxide stimulates type IV MSHA pilus retraction in Vibrio cholerae via activation of the phosphodiesterase CdpA
Abstract
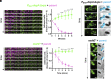
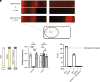
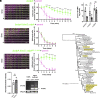
Bacteria use surface appendages called type IV pili to perform diverse activities including DNA uptake, twitching motility, and attachment to surfaces. The dynamic extension and retraction of pili are often required for these activities, but the stimuli that regulate these dynamics remain poorly characterized. To address this question, we study the bacterial pathogen Vibrio cholerae, which uses mannose-sensitive hemagglutinin (MSHA) pili to attach to surfaces in aquatic environments as the first step in biofilm formation. Here, we use a combination of genetic and cell biological approaches to describe a regulatory pathway that allows V. cholerae to rapidly abort biofilm formation. Specifically, we show that V. cholerae cells retract MSHA pili and detach from a surface in a diffusion-limited, enclosed environment. This response is dependent on the phosphodiesterase CdpA, which decreases intracellular levels of cyclic-di-GMP to induce MSHA pilus retraction. CdpA contains a putative nitric oxide (NO)-sensing NosP domain, and we demonstrate that NO is necessary and sufficient to stimulate CdpA-dependent detachment. Thus, we hypothesize that the endogenous production of NO (or an NO-like molecule) in V. cholerae stimulates the retraction of MSHA pili. These results extend our understanding of how environmental cues can be integrated into the complex regulatory pathways that control pilus dynamic activity and attachment in bacterial species.
Keywords: attachment; biofilm; type IV pili.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
C-di-GMP Regulates Motile to Sessile Transition by Modulating MshA Pili Biogenesis and Near-Surface Motility Behavior in Vibrio cholerae.PLoS Pathog. 2015 Oct 27;11(10):e1005068. doi: 10.1371/journal.ppat.1005068. eCollection 2015 Oct. PLoS Pathog. 2015. PMID: 26505896 Free PMC article.
-
c-di-GMP modulates type IV MSHA pilus retraction and surface attachment in Vibrio cholerae.Nat Commun. 2020 Mar 25;11(1):1549. doi: 10.1038/s41467-020-15331-8. Nat Commun. 2020. PMID: 32214098 Free PMC article.
-
The PilT retraction ATPase promotes both extension and retraction of the MSHA type IVa pilus in Vibrio cholerae.PLoS Genet. 2022 Dec 21;18(12):e1010561. doi: 10.1371/journal.pgen.1010561. eCollection 2022 Dec. PLoS Genet. 2022. PMID: 36542674 Free PMC article.
-
Motility and adhesion through type IV pili in Gram-positive bacteria.Biochem Soc Trans. 2016 Dec 15;44(6):1659-1666. doi: 10.1042/BST20160221. Biochem Soc Trans. 2016. PMID: 27913675 Free PMC article. Review.
-
A comprehensive guide to pilus biogenesis in Gram-negative bacteria.Nat Rev Microbiol. 2017 May 12;15(6):365-379. doi: 10.1038/nrmicro.2017.40. Nat Rev Microbiol. 2017. PMID: 28496159 Review.
Cited by
-
Shear force enhances adhesion of Pseudomonas aeruginosa by counteracting pilus-driven surface departure.bioRxiv [Preprint]. 2023 May 8:2023.05.08.539440. doi: 10.1101/2023.05.08.539440. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Oct 10;120(41):e2307718120. doi: 10.1073/pnas.2307718120. PMID: 37215027 Free PMC article. Updated. Preprint.
-
Carbon source, cell density, and the microbial community control inhibition of V. cholerae surface colonization by environmental nitrate.mBio. 2025 Apr 9;16(4):e0406624. doi: 10.1128/mbio.04066-24. Epub 2025 Feb 25. mBio. 2025. PMID: 39998205 Free PMC article.
-
Gas and light: triggers of c-di-GMP-mediated regulation.FEMS Microbiol Rev. 2023 Jul 5;47(4):fuad034. doi: 10.1093/femsre/fuad034. FEMS Microbiol Rev. 2023. PMID: 37339911 Free PMC article. Review.
-
Genomic Insight into Vibrio Isolates from Fresh Raw Mussels and Ready-to-Eat Stuffed Mussels.Pathogens. 2025 Jan 10;14(1):52. doi: 10.3390/pathogens14010052. Pathogens. 2025. PMID: 39861013 Free PMC article.
-
Application of biofilm dispersion-based nanoparticles in cutting off reinfection.Appl Microbiol Biotechnol. 2024 Jun 19;108(1):386. doi: 10.1007/s00253-024-13120-7. Appl Microbiol Biotechnol. 2024. PMID: 38896257 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

