Comparative Effectiveness of mRNA-based BNT162b2 Vaccine versus Adenovirus Vector-Based Ad26.COV2.S Vaccine for the Prevention of COVID-19 among Dialysis Patients
- PMID: 35135894
- PMCID: PMC8970445
- DOI: 10.1681/ASN.2021101395
Comparative Effectiveness of mRNA-based BNT162b2 Vaccine versus Adenovirus Vector-Based Ad26.COV2.S Vaccine for the Prevention of COVID-19 among Dialysis Patients
Abstract
Background: Studies have demonstrated that mRNA-based SARS-CoV-2 vaccines are highly effective among patients on dialysis. Because individual vaccines may be differentially available or acceptable to patients, it is important to understand comparative effectiveness relative to other vaccines, such those on the basis of adenovirus technologies.
Methods: In this retrospective study, we compared the clinical effectiveness of adenovirus vector-based Ad26.COV2.S (Janssen/Johnson & Johnson) to mRNA-based BNT162b2 (Pfizer/BioNTech) in a contemporary cohort of patients on dialysis. Patients who received a first BNT162b2 dose were matched 1:1 to Ad26.COV2.S recipients on the basis of date of first vaccine receipt, US state of residence, site of dialysis care (in-center versus home), history of COVID-19, and propensity score. The primary outcome was the comparative rate of COVID-19 diagnoses starting in the 7th week postvaccination. In a subset of consented patients who received Ad26.COV2.S, blood samples were collected ≥28 days after vaccination and anti-SARS-CoV-2 immunoglobulin G antibodies were measured.
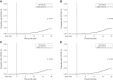
Results: A total of 2572 matched pairs of patients qualified for analysis. Cumulative incidence rates of COVID-19 did not differ for BNT162b2 versus Ad26.COV2.S. No differences were observed in peri-COVID-19 hospitalizations and deaths among patients receiving BNT162b2 versus Ad26.COV2.S, who were diagnosed with COVID-19 during the at-risk period. Results were similar when excluding patients with a history of COVID-19, in subgroup analyses restricted to patients who completed the two-dose BNT162b2 regimen, and in patients receiving in-center hemodialysis. SARS-CoV-2 antibodies were detected in 59.4% of 244 patients who received Ad26.COV2.S.
Conclusions: In a large real-world cohort of patients on dialysis, no difference was detected in clinical effectiveness of BNT162b2 and Ad26.COV2.S over the first 6 months postvaccination, despite an inconsistent antibody response to the latter.
Keywords: BNT162 vaccine; COVID-19; SARS-CoV-2; comparative effectiveness; dialysis; vaccine.
Copyright © 2022 by the American Society of Nephrology.
Figures


References
-
- Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC: Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 324: 782–793, 2020 - PubMed
-
- Center for Medicare and Medicaid Services : Preliminary Medicare COVID-19 data snapshot. Available at: https://www.cms.gov/files/document/medicare-covid-19-data-snapshot-fact-.... Accessed August 1, 2021
-
- Centers for Disease Control and Prevention : COVID-19 vaccine: What public health jurisdictions and dialysis partners need to know. Available at: https://www.cdc.gov/vaccines/covid-19/planning/dialysis-partners-jurisdi.... Accessed June 17, 2021
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

