Evaluation of a D-Octaarginine-linked polymer as a transfection tool for transient and stable transgene expression in human and murine cell lines
- PMID: 35135938
- PMCID: PMC9096039
- DOI: 10.1292/jvms.21-0647
Evaluation of a D-Octaarginine-linked polymer as a transfection tool for transient and stable transgene expression in human and murine cell lines
Abstract
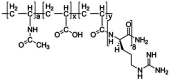
Poly(N-vinylacetamide-co-acrylic acid) coupled with d-octaarginine (VP-R8) promotes the cellular uptake of peptides/proteins in vitro; however, details of the transfection efficacy of VP-R8, such as the cell types possessing high gene transfer, are not known. Herein, we compared the ability of VP-R8 to induce the cellular uptake of plasmid DNA in mouse and human cell lines from different tissues and organs. A green fluorescent protein (GFP)-expression plasmid was used as model genetic material, and fluorescence as an indicator of uptake and plasmid-derived protein expression. Three mouse and three human cell lines were incubated with a mixture of plasmid and VP-R8, and fluorescence analysis were performed two days after transfection. To confirm stable transgene expression, we performed drug selection three days after transfection. A commercially available polymer-based DNA transfection reagent (PTR) was used as the transfection control and standard for comparing transgene expression efficiency. In the case of transient transgene expression, slight-to-moderate GFP expression was observed in all cell lines transfected with plasmid via VP-R8; however, transfection efficiency was lower than using the PTR for gene delivery. In the case of stable transgene expression, VP-R8 promoted drug-resistance acquisition more efficiently than the PTR did. Cells that developed drug resistance after VP-R8-mediated gene transfection expressed GFP more efficiently than cells that developed drug resistance after transfection with the PTR. Thus, VP-R8 shows potential as an in vitro or ex vivo nonviral transfection tool for generating cell lines with stable transgene expression.
Keywords: gene-delivery; plasmid-derived protein expression; poly(N-vinylacetamide-co-acrylic acid) bearing d-octaarginine; stable transgene expression; transient transgene expression.
Figures

Similar articles
-
The Improved Antigen Uptake and Presentation of Dendritic Cells Using Cell-Penetrating D-octaarginine-Linked PNVA-co-AA as a Novel Dendritic Cell-Based Vaccine.Int J Mol Sci. 2024 May 30;25(11):5997. doi: 10.3390/ijms25115997. Int J Mol Sci. 2024. PMID: 38892182 Free PMC article.
-
Potential of D-Octaarginine-Linked Polymers as an in Vitro Transfection Tool for Biomolecules.Bioconjug Chem. 2015 Aug 19;26(8):1782-90. doi: 10.1021/acs.bioconjchem.5b00323. Epub 2015 Aug 7. Bioconjug Chem. 2015. PMID: 26252905
-
Hyaluronic acid controls the uptake pathway and intracellular trafficking of an octaarginine-modified gene vector in CD44 positive- and CD44 negative-cells.Biomaterials. 2015 Jun;52:189-98. doi: 10.1016/j.biomaterials.2015.02.027. Epub 2015 Feb 25. Biomaterials. 2015. PMID: 25818425
-
[Development of a novel artificial gene delivery system multifunctional envelope-type nano device for gene therapy].Yakugaku Zasshi. 2007 Oct;127(10):1685-91. doi: 10.1248/yakushi.127.1685. Yakugaku Zasshi. 2007. PMID: 17917425 Review. Japanese.
-
In situ transient transfection of 3D cell cultures and tissues, a promising tool for tissue engineering and gene therapy.Biotechnol Adv. 2023 Nov;68:108211. doi: 10.1016/j.biotechadv.2023.108211. Epub 2023 Jul 16. Biotechnol Adv. 2023. PMID: 37463610 Review.
Cited by
-
G-protein-coupled estrogen receptor prevents nuclear factor-kappa B promoter activation by Helicobacter pylori cytotoxin-associated gene A in gastric cancer cells.J Vet Med Sci. 2023 Dec 27;85(12):1348-1354. doi: 10.1292/jvms.23-0054. Epub 2023 Nov 13. J Vet Med Sci. 2023. PMID: 37952974 Free PMC article.
-
The Improved Antigen Uptake and Presentation of Dendritic Cells Using Cell-Penetrating D-octaarginine-Linked PNVA-co-AA as a Novel Dendritic Cell-Based Vaccine.Int J Mol Sci. 2024 May 30;25(11):5997. doi: 10.3390/ijms25115997. Int J Mol Sci. 2024. PMID: 38892182 Free PMC article.
References
-
- Bradbury A. M., Cochran J. N., McCurdy V. J., Johnson A. K., Brunson B. L., Gray-Edwards H., Leroy S. G., Hwang M., Randle A. N., Jackson L. S., Morrison N. E., Baek R. C., Seyfried T. N., Cheng S. H., Cox N. R., Baker H. J., Cachón-González M. B., Cox T. M., Sena-Esteves M., Martin D. R.2013. Therapeutic response in feline sandhoff disease despite immunity to intracranial gene therapy. Mol. Ther. 21: 1306–1315. doi: 10.1038/mt.2013.86 - DOI - PMC - PubMed
-
- Colosimo A., Goncz K. K., Holmes A. R., Kunzelmann K., Novelli G., Malone R. W., Bennett M. J., Gruenert D. C.2000. Transfer and expression of foreign genes in mammalian cells. Biotechniques 29: 314–318, 320,–312, 324. - PubMed
-
- Du L. M., Nurden P., Nurden A. T., Nichols T. C., Bellinger D. A., Jensen E. S., Haberichter S. L., Merricks E., Raymer R. A., Fang J., Koukouritaki S. B., Jacobi P. M., Hawkins T. B., Cornetta K., Shi Q., Wilcox D. A.2013. Platelet-targeted gene therapy with human factor VIII establishes haemostasis in dogs with haemophilia A. Nat. Commun. 4: 2773. doi: 10.1038/ncomms3773 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

