The C-type lectin receptor Clec1A plays an important role in the development of experimental autoimmune encephalomyelitis by enhancing antigen presenting ability of dendritic cells and inducing inflammatory cytokine IL-17
- PMID: 35135958
- PMCID: PMC9388343
- DOI: 10.1538/expanim.21-0191
The C-type lectin receptor Clec1A plays an important role in the development of experimental autoimmune encephalomyelitis by enhancing antigen presenting ability of dendritic cells and inducing inflammatory cytokine IL-17
Abstract
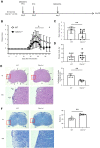
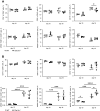
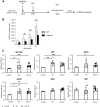
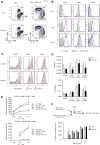
Clec1A, a member of C-type lectin receptor family, has a carbohydrate recognition domain in its extracellular region, but no known signaling motif in the cytoplasmic domain. Clec1a is highly expressed in endothelial cells and weakly in dendritic cells. Although this molecule was reported to play an important role in the host defense against Aspergillus fumigatus by recognizing 1,8-dihydroxynaphthalene-melanin on the fungal surface, the roles of this molecule in un-infected animals remain to be elucidated. In this study, we found that Clec1a-/- mice develop milder symptoms upon induction of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis. The maximum disease score was significantly lower, and demyelination and inflammation of the spinal cord were much milder in Clec1a-/- mice compared to wild-type mice. No abnormality was detected in the immune cell composition in the draining lymph nodes and spleen on day 10 and 16 after EAE induction. Recall memory T cell proliferation after restimulation with myelin oligodendrocyte glycoprotein peptide (MOG35-55) in vitro was decreased in Clec1a-/- mice, and antigen presenting ability of Clec1a-/- dendritic cells was impaired. Interestingly, RNA-Seq and RT-qPCR analyses clearly showed that the expression of inflammatory cytokines including Il17a, Il6 and Il1b was greatly decreased in Clec1a-/- mice after induction of EAE, suggesting that this reduced cytokine production is responsible for the amelioration of EAE in Clec1a-/- mice. These observations suggest a novel function of Clec1A in the immune system.
Keywords: C-type lectin receptor; Clec1a; IL-17; experimental autoimmune encephalomyelitis.
Figures

Similar articles
-
Myelin oligodendrocyte glycoprotein (MOG35-55)-induced experimental autoimmune encephalomyelitis is ameliorated in interleukin-32 alpha transgenic mice.Oncotarget. 2015 Dec 1;6(38):40452-63. doi: 10.18632/oncotarget.6306. Oncotarget. 2015. PMID: 26564962 Free PMC article.
-
Clinical and immunological control of experimental autoimmune encephalomyelitis by tolerogenic dendritic cells loaded with MOG-encoding mRNA.J Neuroinflammation. 2019 Aug 15;16(1):167. doi: 10.1186/s12974-019-1541-1. J Neuroinflammation. 2019. PMID: 31416452 Free PMC article.
-
Lipocalin-2 protein deficiency ameliorates experimental autoimmune encephalomyelitis: the pathogenic role of lipocalin-2 in the central nervous system and peripheral lymphoid tissues.J Biol Chem. 2014 Jun 13;289(24):16773-89. doi: 10.1074/jbc.M113.542282. Epub 2014 May 7. J Biol Chem. 2014. PMID: 24808182 Free PMC article.
-
Tolerogenic dendritic cells produced by lentiviral-mediated CD40- and interleukin-23p19-specific shRNA can ameliorate experimental autoimmune encephalomyelitis by suppressing T helper type 17 cells.Clin Exp Immunol. 2014 May;176(2):180-9. doi: 10.1111/cei.12266. Clin Exp Immunol. 2014. PMID: 24387596 Free PMC article.
-
RGS10 deficiency ameliorates the severity of disease in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2016 Feb 1;13:24. doi: 10.1186/s12974-016-0491-0. J Neuroinflammation. 2016. PMID: 26831924 Free PMC article.
Cited by
-
Single-Cell Analysis of Host Responses in Bovine Milk Somatic Cells (bMSCs) Following HPAIV Bovine H5N1 Influenza Exposure.Viruses. 2025 Jun 3;17(6):811. doi: 10.3390/v17060811. Viruses. 2025. PMID: 40573402 Free PMC article.
-
The effect of psychoactive bacteria, Bifidobacterium longum Rosell®-175 and Lactobacillus rhamnosus JB-1, on brain proteome profiles in mice.Psychopharmacology (Berl). 2024 May;241(5):925-945. doi: 10.1007/s00213-023-06519-z. Epub 2023 Dec 29. Psychopharmacology (Berl). 2024. PMID: 38156998 Free PMC article.
-
Antithrombin regulates neutrophil activities through the stimulation of C-type lectin family 1A.Blood Vessel Thromb Hemost. 2024 Oct 8;2(1):100032. doi: 10.1016/j.bvth.2024.100032. eCollection 2025 Feb. Blood Vessel Thromb Hemost. 2024. PMID: 40766868 Free PMC article.

