Role of mTORC1 activity during early retinal development and lamination in human-induced pluripotent stem cell-derived retinal organoids
- PMID: 35136019
- PMCID: PMC8826382
- DOI: 10.1038/s41420-022-00837-5
Role of mTORC1 activity during early retinal development and lamination in human-induced pluripotent stem cell-derived retinal organoids
Abstract
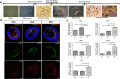
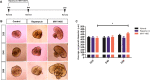
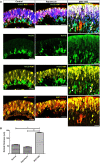
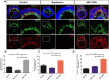
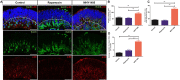
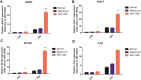
Retinal organoids derived from human-induced pluripotent stem cells (hiPSC) are powerful tools for studying retinal development as they model spatial and temporal differentiation of retinal cell types. Vertebrate retinal development involves a delicate and coordinated process of retinal progenitor cell (RPC) differentiation, and the mammalian target of rapamycin complex 1 (mTORC1) has been reported to play a significant role in this complex process. Herein, using hiPSC-derived retinal organoids, we identify the time-dependent role of mTORC1 in retinal development, specifically in retinal ganglion cell (RGC) differentiation and the retinal lamination process, during the early stages of retinal organoid (RO) development. mTORC1 activity in ROs was the highest at 40 days of differentiation. MHY1485-induced hyperactivation of mTORC1 during this period resulted in a significant increase in the overall size of ROs compared to the untreated controls and rapamycin-treated Ros; there was also a marked increase in proliferative activity within the inner and outer layers of ROs. Moreover, the MHY1485-treated ROs showed a significant increase in the number of ectopic RGCs in the outer layers (indicating disruption of retinal laminar structure), with robust expression of HuC/D-binding proteins in the inner layers. These results demonstrate that mTORC1 plays a critical role in the development of hiPSC-derived ROs, especially during the early stages of differentiation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Grants and funding
- 2019R1A2C1005055/Ministry of Education (Ministry of Education of the Republic of Korea)
- 2020R1I1A3053309/Ministry of Education (Ministry of Education of the Republic of Korea)
- HI21C0317/Ministry of Health and Welfare (Ministry of Health, Welfare and Family Affairs)
- 20009844/Ministry of Trade, Industry and Energy (Ministry of Trade, Industry and Energy, Korea)
LinkOut - more resources
Full Text Sources
Research Materials

