Targeted activation of androgen receptor signaling in the periosteum improves bone fracture repair
- PMID: 35136023
- PMCID: PMC8826926
- DOI: 10.1038/s41419-022-04595-1
Targeted activation of androgen receptor signaling in the periosteum improves bone fracture repair
Abstract
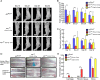
Low testosterone level is an independent predictor of osteoporotic fracture in elderly men as well as increased fracture risk in men undergoing androgen deprivation. Androgens and androgen receptor (AR) actions are essential for bone development and homeostasis but their linkage to fracture repair remains unclear. Here we found that AR is highly expressed in the periosteum cells and is co-localized with a mesenchymal progenitor cell marker, paired-related homeobox protein 1 (Prrx1), during bone fracture repair. Mice lacking the AR gene in the periosteum expressing Prrx1-cre (AR-/Y;Prrx1::Cre) but not in the chondrocytes (AR-/Y;Col-2::Cre) exhibits reduced callus size and new bone volume. Gene expression data analysis revealed that the expression of several collagens, integrins and cell adhesion molecules were downregulated in periosteum-derived progenitor cells (PDCs) from AR-/Y;Prrx1::Cre mice. Mechanistically, androgens-AR signaling activates the AR/ARA55/FAK complex and induces the collagen-integrin α2β1 gene expression that is required for promoting the AR-mediated PDCs migration. Using mouse cortical-defect and femoral graft transplantation models, we proved that elimination of AR in periosteum of host mice impairs fracture healing, regardless of AR existence of transplanted donor graft. While testosterone implanted scaffolds failed to complete callus bridging across the fracture gap in AR-/Y;Prrx1::Cre mice, cell-based transplantation using DPCs re-expressing AR could lead to rescue bone repair. In conclusion, targeting androgen/AR axis in the periosteum may provide a novel therapy approach to improve fracture healing.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Murao H, Yamamoto K, Matsuda S, Akiyama H. Periosteal cells are a major source of soft callus in bone fracture. J Bone Min Metab. 2013;31:390–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NMRPG8E0351/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)
- NMRPD1E0611-3/Ministry of Science and Technology, Taiwan (Ministry of Science and Technology of Taiwan)
- CMRPD8G0081-3/Chang Gung Memorial Hospital (CGMH)
- CMRPD8D0472-3/Chang Gung Memorial Hospital (CGMH)
- CMRPD8D0071-2/Chang Gung Medical Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

