LncRNA BC200/miR-150-5p/MYB positive feedback loop promotes the malignant proliferation of myelodysplastic syndrome
- PMID: 35136029
- PMCID: PMC8825806
- DOI: 10.1038/s41419-022-04578-2
LncRNA BC200/miR-150-5p/MYB positive feedback loop promotes the malignant proliferation of myelodysplastic syndrome
Abstract
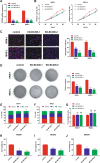
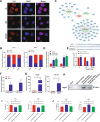
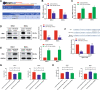
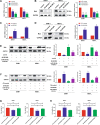
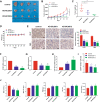
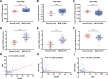
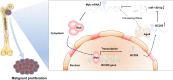
Myelodysplastic syndrome (MDS) is a group of heterogeneous hematologic malignancies with a risk of transformation to acute myeloid leukemia. Understanding the molecular mechanisms of the specific roles of long noncoding RNAs (lncRNAs) in MDS would create novel ways to identify diagnostic and therapeutic targets. The lncRNA BC200 is upregulated and acts as an oncogene in various cancers; however, its expression, clinical significance, and roles in MDS remain unclear. Here, we found that BC200 was highly expressed in MDS patients compared with normal individuals. Knockdown of BC200 inhibited MDS cell proliferation, colony formation, and cell cycle progression in vitro and suppressed the growth and invasiveness of MDS cells in vivo. Mechanistic investigations revealed that BC200 functioned as a miRNA sponge to positively regulate the expression of MYB through sponging miR-150-5p and subsequently promoted malignant proliferation of MDS cells. Conversely, we found that BC200 was a direct transcriptional target of MYB, and knockdown of MYB abolished the oncogenic effect of BC200/miR-150-5p. Taken together, our results revealed that the BC200/miR-150-5p/MYB positive feedback loop promoted the proliferation of MDS cells and is expected to be a potential biomarker and therapeutic target in MDS.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Steensma DP. Myelodysplastic syndromes: diagnosis and treatment. Mayo Clin Proc. 2015;90:969–83.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 82003286/National Natural Science Foundation of China (National Science Foundation of China)
- 81770107, 81920108004/National Natural Science Foundation of China (National Science Foundation of China)
- 81970195/National Natural Science Foundation of China (National Science Foundation of China)
- 81870105/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

