The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders
- PMID: 35136038
- PMCID: PMC8825858
- DOI: 10.1038/s41419-022-04593-3
The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders
Abstract
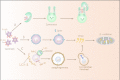
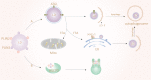
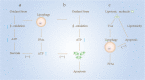
Autophagy is a conserved method of quality control in which cytoplasmic contents are degraded via lysosomes. Lipophagy, a form of selective autophagy and a novel type of lipid metabolism, has recently received much attention. Lipophagy is defined as the autophagic degradation of intracellular lipid droplets (LDs). Although much remains unknown, lipophagy appears to play a significant role in many organisms, cell types, metabolic states, and diseases. It participates in the regulation of intracellular lipid storage, intracellular free lipid levels (e.g., fatty acids), and energy balance. However, it remains unclear how intracellular lipids regulate autophagy. Impaired lipophagy can cause cells to become sensitive to death stimuli and may be responsible for the onset of a variety of diseases, including nonalcoholic fatty liver disease and metabolic syndrome. Like autophagy, the role of lipophagy in cancer is poorly understood, although analysis of specific autophagy receptors has helped to expand the diversity of chemotherapeutic targets. These studies have stimulated increasing interest in the role of lipophagy in the pathogenesis and treatment of cancer and other human diseases.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–64.. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

