Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice
- PMID: 35136064
- PMCID: PMC8825811
- DOI: 10.1038/s41467-022-28131-z
Amylin-Calcitonin receptor signaling in the medial preoptic area mediates affiliative social behaviors in female mice
Abstract
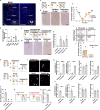
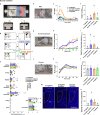
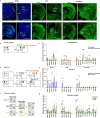
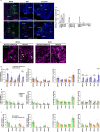
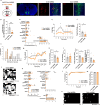
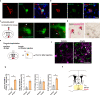
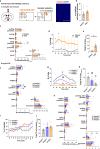
Social animals actively engage in contact with conspecifics and experience stress upon isolation. However, the neural mechanisms coordinating the sensing and seeking of social contacts are unclear. Here we report that amylin-calcitonin receptor (Calcr) signaling in the medial preoptic area (MPOA) mediates affiliative social contacts among adult female mice. Isolation of females from free social interactions first induces active contact-seeking, then depressive-like behavior, concurrent with a loss of Amylin mRNA expression in the MPOA. Reunion with peers induces physical contacts, activates both amylin- and Calcr-expressing neurons, and leads to a recovery of Amylin mRNA expression. Chemogenetic activation of amylin neurons increases and molecular knockdown of either amylin or Calcr attenuates contact-seeking behavior, respectively. Our data provide evidence in support of a previously postulated origin of social affiliation in mammals.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. - PubMed
-
- Poole, T. B. Social behaviour in mammals (Blackie; Distributed in the USA by Chapman and Hall, Glasgow, New York, 1985).
-
- Wilson, E. O. Sociobiology: the new synthesis (Belknap Press, 1975).
-
- Rubenstein, D. R. & Abbot, P. Comparative social evolution (Cambridge University Press, Cambridge, United Kingdom, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

