Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: a systematic review and meta-analysis
- PMID: 35136066
- PMCID: PMC8826852
- DOI: 10.1038/s41598-022-05682-1
Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: a systematic review and meta-analysis
Abstract
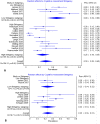
Breast cancer survival rates have markedly improved. Consequently, survivorship issues have received increased attention. One common sequel of treatment is chemotherapy-induced cognitive impairment (CICI). CICI causes a range of impairments that can have a significant negative impact on quality of life. Knowledge of the prevalence of this condition is required to inform survivorship plans, and ensure adequate resource allocation and support is available for sufferers, hence a systematic review of prevalence data was performed. Medline, Scopus, CINAHL and PSYCHInfo were searched for eligible studies which included prevalence data on CICI, as ascertained though the use of self-report, or neuropsychological tests. Methodological quality of included studies was assessed. Findings were synthesised narratively, with meta-analyses being used to calculate pooled prevalence when impairment was assessed by neuropsychological tests. The review included 52 studies. Time-points considered ranged from the chemotherapy treatment period to greater than 10 years after treatment cessation. Summary prevalence figures (across time-points) using self-report, short cognitive screening tools and neuropsychological test batteries were 44%, 16% and 21-34% respectively (very low GRADE evidence). Synthesised findings demonstrate that 1 in 3 breast cancer survivors may have clinically significant cognitive impairment. Prevalence is higher when self-report based on patient experience is considered. This review highlights a number of study design issues that may have contributed to the low certainty rating of the evidence. Future studies should take a more consistent approach to the criteria used to assess impairment. Larger studies are urgently needed.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
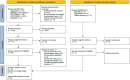
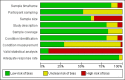
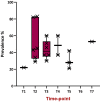
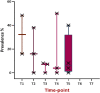


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

