An extended motif in the SARS-CoV-2 spike modulates binding and release of host coatomer in retrograde trafficking
- PMID: 35136165
- PMCID: PMC8825798
- DOI: 10.1038/s42003-022-03063-y
An extended motif in the SARS-CoV-2 spike modulates binding and release of host coatomer in retrograde trafficking
Abstract
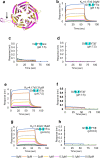
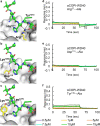
β-Coronaviruses such as SARS-CoV-2 hijack coatomer protein-I (COPI) for spike protein retrograde trafficking to the progeny assembly site in endoplasmic reticulum-Golgi intermediate compartment (ERGIC). However, limited residue-level details are available into how the spike interacts with COPI. Here we identify an extended COPI binding motif in the spike that encompasses the canonical K-x-H dibasic sequence. This motif demonstrates selectivity for αCOPI subunit. Guided by an in silico analysis of dibasic motifs in the human proteome, we employ mutagenesis and binding assays to show that the spike motif terminal residues are critical modulators of complex dissociation, which is essential for spike release in ERGIC. αCOPI residues critical for spike motif binding are elucidated by mutagenesis and crystallography and found to be conserved in the zoonotic reservoirs, bats, pangolins, camels, and in humans. Collectively, our investigation on the spike motif identifies key COPI binding determinants with implications for retrograde trafficking.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. - PubMed
-
- USFDA. U.S. Food and Drug Administration. Moderna COVID-19 vaccine [FDA briefing document]. Silver Spring, MD: U.S. Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee; 2020, https://www.fda.gov/media/144434/download (2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

