Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective
- PMID: 35136306
- PMCID: PMC8814804
- DOI: 10.1007/s41745-021-00268-8
Neutralizing Antibodies and Antibody-Dependent Enhancement in COVID-19: A Perspective
Abstract
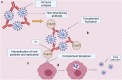
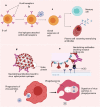
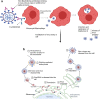
Antibody-dependent enhancement (ADE) is an alternative route of viral entry in the susceptible host cell. In this process, antiviral antibodies enhance the entry access of virus in the cells via interaction with the complement or Fc receptors leading to the worsening of infection. SARS-CoV-2 variants pose a general concern for the efficacy of neutralizing antibodies that may fail to neutralize infection, raising the possibility of a more severe form of COVID-19. Data from various studies on respiratory viruses raise the speculation that antibodies elicited against SARS-CoV-2 and during COVID-19 recovery could potentially exacerbate the infection through ADE at sub-neutralizing concentrations; this may contribute to disease pathogenesis. It is, therefore, of utmost importance to study the effectiveness of the anti-SARS-CoV-2 antibodies in COVID-19-infected subjects. Theoretically, ADE remains a general concern for the efficacy of antibodies elicited during infection, most notably in convalescent plasma therapy and in response to vaccines where it could be counterproductive.
Keywords: ADE; ARDS; ERD; Enhancement; Neutralizing antibodies; SARS-CoV-2.
© Indian Institute of Science 2022.
Conflict of interest statement
Conflict of interestThe authors report no declarations of interest.
Figures

Similar articles
-
Multiple Routes of Antibody-Dependent Enhancement of SARS-CoV-2 Infection.Microbiol Spectr. 2022 Apr 27;10(2):e0155321. doi: 10.1128/spectrum.01553-21. Epub 2022 Mar 23. Microbiol Spectr. 2022. PMID: 35319248 Free PMC article.
-
Neutralizing antibodies from the rare convalescent donors elicited antibody-dependent enhancement of SARS-CoV-2 variants infection.Front Med (Lausanne). 2022 Oct 19;9:952697. doi: 10.3389/fmed.2022.952697. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36341247 Free PMC article.
-
Antibody-Dependent Enhancement of SARS-CoV-2 Infection Is Mediated by the IgG Receptors FcγRIIA and FcγRIIIA but Does Not Contribute to Aberrant Cytokine Production by Macrophages.mBio. 2021 Oct 26;12(5):e0198721. doi: 10.1128/mBio.01987-21. Epub 2021 Sep 28. mBio. 2021. PMID: 34579572 Free PMC article.
-
Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis.Mol Immunol. 2022 Dec;152:172-182. doi: 10.1016/j.molimm.2022.11.010. Epub 2022 Nov 10. Mol Immunol. 2022. PMID: 36371813 Free PMC article. Review.
-
Antibody-Dependent Enhancement with a Focus on SARS-CoV-2 and Anti-Glycan Antibodies.Viruses. 2023 Jul 20;15(7):1584. doi: 10.3390/v15071584. Viruses. 2023. PMID: 37515270 Free PMC article. Review.
Cited by
-
Structural Dynamics of the SARS-CoV-2 Spike Protein: A 2-Year Retrospective Analysis of SARS-CoV-2 Variants (from Alpha to Omicron) Reveals an Early Divergence between Conserved and Variable Epitopes.Molecules. 2022 Jun 15;27(12):3851. doi: 10.3390/molecules27123851. Molecules. 2022. PMID: 35744971 Free PMC article.
-
Antibody-dependent enhancement of SARS-CoV-2, the impact of variants and vaccination.Hum Vaccin Immunother. 2025 Dec;21(1):2505356. doi: 10.1080/21645515.2025.2505356. Epub 2025 May 24. Hum Vaccin Immunother. 2025. PMID: 40411306 Free PMC article.
-
SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: Antibody-dependent as well as independent cell entry.Front Immunol. 2022 Dec 1;13:1050478. doi: 10.3389/fimmu.2022.1050478. eCollection 2022. Front Immunol. 2022. PMID: 36532011 Free PMC article. Review.
-
Efficacy and safety of SARS-CoV-2 neutralizing antibody, SCTA01, in high-risk outpatients diagnosed with COVID-19: A Phase II clinical trial.Contemp Clin Trials Commun. 2025 May 17;45:101496. doi: 10.1016/j.conctc.2025.101496. eCollection 2025 Jun. Contemp Clin Trials Commun. 2025. PMID: 40520909 Free PMC article.
-
Pediatric multisystem inflammatory syndrome associated with COVID-19: Insights in pathogenesis and clinical management.World J Virol. 2023 Jun 25;12(3):193-203. doi: 10.5501/wjv.v12.i3.193. World J Virol. 2023. PMID: 37396702 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
