Spinal Dysraphisms: A New Anatomical-Clinicoradiological Classification
- PMID: 35136492
- PMCID: PMC8817824
- DOI: 10.1055/s-0041-1741100
Spinal Dysraphisms: A New Anatomical-Clinicoradiological Classification
Abstract
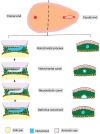
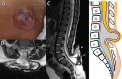
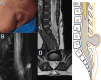
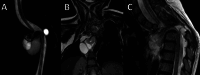
Background Spinal dysraphisms refer to the congenital abnormalities of the spine and spinal cord due to aberrations in the processes of gastrulation, primary neurulation, and secondary neurulation. Embryology of many complex spinal dysraphisms are yet poorly understood and there is no agreeable anatomical-clinicoradiological classification with inclusion of recently documented and complex spinal dysraphisms. Aims and Objectives The main objective of this study was to review the imaging features of spinal dysraphisms with a better understanding of embryological abnormalities and propose a new classification inclusive of all complex and unusual dysraphisms based on anatomical and clinicoradiological correlation. Materials and Methods This was a retrospective single institutional observational study of 391 cases of spinal dysraphism for 10 years in our institution. Of 391 cases included in the study, 204 were males and 187 were females. Also, 123 cases belonged to the 0-6 months age group, 38 cases belonged to the 7-12 months age group, 156 belonged to the 1-5 years age group, 39 cases belonged to the 6-10 years age group, and 35 cases belonged to 10-20 years age group. Results An anatomical-clinicoradiological analysis of cases yielded a high proportion of cases of spinal lipomas, including lipomyeloceles and lipomyelomeningoceles (31.3%) and posterior myelomeningocele (14.2%). Anterior myelocoele (0.2%), sacral chordoma(0.2%), and intrasacral meningocele (0.2%) formed the least proportion of cases. A new classification was proposed based on the analysis of acquired data. Conclusion A structured approach in imaging spinal dysraphism is necessary for imaging evaluation in recent years. The proposed new classification based on clinicoradiological correlation and anatomic location is inclusive of unusual and complex dysraphisms.
Keywords: closed spinal dysraphism; embryology; magnetic resonance imaging; open spinal dysraphism; spinal dysraphism.
Indian Radiological Association. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. ( https://creativecommons.org/licenses/by-nc-nd/4.0/ ).
Figures








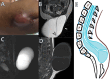
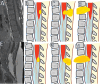
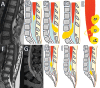

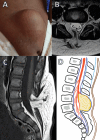
















References
-
- Tortori-Donati P, Rossi A, Cama A. Spinal dysraphism: a review of neuroradiological features with embryological correlations and proposal for a new classification. Neuroradiology. 2000;42(07):471–491. - PubMed
-
- Sadler T W. Embryology of neural tube development. Am J Med Genet C Semin Med Genet. 2005;135C(01):2–8. - PubMed
-
- Moore K. Philadelphia, PA: Elsevier Saunders; 2013. Congenital Abnormalities of the Spine; pp. 449–60.
-
- NTD Collaborative Group . Melvin E C, George T M, Worley G et al. Genetic studies in neural tube defects. Pediatr Neurosurg. 2000;32(01):1–9. - PubMed
LinkOut - more resources
Full Text Sources

