Sampling Validation Data to Achieve a Planned Precision of the Bias-Adjusted Estimate of Effect
- PMID: 35136909
- PMCID: PMC9989334
- DOI: 10.1093/aje/kwac025
Sampling Validation Data to Achieve a Planned Precision of the Bias-Adjusted Estimate of Effect
Abstract
Data collected from a validation substudy permit calculation of a bias-adjusted estimate of effect that is expected to equal the estimate that would have been observed had the gold standard measurement been available for the entire study population. In this paper, we develop and apply a framework for adaptive validation to determine when sufficient validation data have been collected to yield a bias-adjusted effect estimate with a prespecified level of precision. Prespecified levels of precision are decided a priori by the investigator, based on the precision of the conventional estimate and allowing for wider confidence intervals that would still be substantively meaningful. We further present an applied example of the use of this method to address exposure misclassification in a study of transmasculine/transfeminine youth and self-harm. Our method provides a novel approach to effective and efficient estimation of classification parameters as validation data accrue, with emphasis on the precision of the bias-adjusted estimate. This method can be applied within the context of any parent epidemiologic study design in which validation data will be collected and modified to meet alternative criteria given specific study or validation study objectives.
Keywords: epidemiologic methods; quantitative bias analysis; study design; validation substudies.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


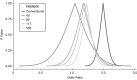

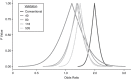
References
-
- Rothman KJ, Greenland S. Planning study size based on precision rather than power. Epidemiology. 2018;29(5):599–603. - PubMed
-
- von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. - PubMed
-
- Holcroft CA, Spiegelman D. Design of validation studies for estimating the odds ratio of exposure-disease relationships when exposure is misclassified. Biometrics. 1999;55(4):1193–1201. - PubMed
-
- Spiegelman D, Rosner B, Logan R. Estimation and inference for logistic regression with covariate misclassification and measurement error in main study/validation study designs. J Am Stat Assoc. 2000;95(449):51–61.
-
- Greenland S. Variance estimation for epidemiologic effect estimates under misclassification. Stat Med. 1988;7(7):745–757. - PubMed

