Characterisation of the E. coli and Salmonella qseC and qseE mutants reveals a metabolic rather than adrenergic receptor role
- PMID: 35137015
- PMCID: PMC8897314
- DOI: 10.1093/femsle/fnac012
Characterisation of the E. coli and Salmonella qseC and qseE mutants reveals a metabolic rather than adrenergic receptor role
Abstract
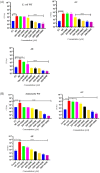
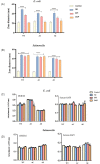
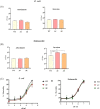
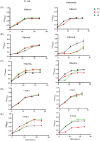
Catecholamine stress hormones (norepinephrine, epinephrine, and dopamine) are signals that have been shown to be used as environmental cues, which affect the growth and virulence of normal microbiota as well as pathogenic bacteria. It has been reported that Escherichia coli and Salmonella use the two-component system proteins QseC and QseE to recognise catecholamines and so act as bacterial adrenergic receptors. In this study, we mutated the E. coli O157:H7 and Salmonella enterica serovar Typhimurium genes encoding QseC and QseE and found that this did not block stress hormone responsiveness in either species. Motility, biofilm formation, and analysis of virulence of the mutants using two infection models were similar to the wild-type strains. The main differences in phenotypes of the qseC and qseE mutants were responses to changes in temperature and growth in different media particularly with respect to salt, carbon, and nitrogen salt sources. In this physiological respect, it was also found that the phenotypes of the qseC and qseE mutants differed between E. coli and Salmonella. These findings collectively suggest that QseC and QseE are not essential for E. coli and Salmonella to respond to stress hormones and that the proteins may be playing a role in regulating metabolism.
Keywords: E. coli; Salmonella; QseC; QseE; catecholamines; stress.
© The Author(s) 2022. Published by Oxford University Press on behalf of FEMS.
Figures


References
-
- Bearson BL, Bearson SM, Lee ISet al. . The Salmonellaenterica serovar Typhimurium QseB response regulator negatively regulates bacterial motility and swine colonization in the absence of the QseC sensor kinase. Microb Pathog. 2010;48:214–9. - PubMed
-
- Bearson BL, Bearson SM, Uthe JJet al. . Iron regulated genes of Salmonellaenterica serovar Typhimurium in response to norepinephrine and the requirement of fepDGC for norepinephrine enhanced growth. Microbes Infect. 2008;10:807–16. - PubMed
-
- Borre YE, Moloney RD, Clarke Get al. . The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. In: Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. New York: Springer, 2014, 373–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

